Fluorine facts for kids

Liquid fluorine (at extremely low temperatures)
|
|||||||||||||||||||||
| Fluorine | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | |||||||||||||||||||||
| Allotropes | alpha, beta (see Allotropes of fluorine) | ||||||||||||||||||||
| Appearance | gas: very pale yellow liquid: bright yellow solid: transparent (beta), opaque (alpha) |
||||||||||||||||||||
| Standard atomic weight Ar, std(F) | 18.998403163(6) | ||||||||||||||||||||
| Fluorine in the periodic table | |||||||||||||||||||||
|
|||||||||||||||||||||
| Atomic number (Z) | 9 | ||||||||||||||||||||
| Group | group 17 (halogens) | ||||||||||||||||||||
| Period | period 2 | ||||||||||||||||||||
| Block | p | ||||||||||||||||||||
| Electron configuration | [He] 2s2 2p5 | ||||||||||||||||||||
| Electrons per shell | 2, 7 | ||||||||||||||||||||
| Physical properties | |||||||||||||||||||||
| Phase at STP | gas | ||||||||||||||||||||
| Melting point | 53.53 K (−219.62 °C, −363.32 °F) | ||||||||||||||||||||
| Boiling point | 85.03 K (−188.12 °C, −306.62 °F) | ||||||||||||||||||||
| Density (at STP) | 1.696 g/L | ||||||||||||||||||||
| when liquid (at b.p.) | 1.505 g/cm3 | ||||||||||||||||||||
| Critical point | 144.4 K, 5.215 MPa | ||||||||||||||||||||
| Heat of vaporization | 6.51 kJ/mol | ||||||||||||||||||||
| Molar heat capacity | (Cp) (21.1 °C) 825 J·mol−1·K−1 (Cv) (21.1 °C) 610 J/(mol·K) |
||||||||||||||||||||
Vapor pressure
|
|||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||
| Oxidation states | −1 (oxidizes oxygen) | ||||||||||||||||||||
| Electronegativity | Pauling scale: 3.98 | ||||||||||||||||||||
| Ionization energies |
|
||||||||||||||||||||
| Covalent radius | 64 pm | ||||||||||||||||||||
| Van der Waals radius | 135 pm | ||||||||||||||||||||
| Spectral lines of fluorine | |||||||||||||||||||||
| Other properties | |||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||
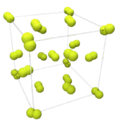
| Crystal structure | cubic
the structure shows solid fluorine, just under the melting point, 1 atm |
||||||||||||||||||||
| Thermal conductivity | 0.02591 W/(m⋅K) | ||||||||||||||||||||
| Magnetic ordering | diamagnetic | ||||||||||||||||||||
| CAS Number | 7782-41-4 | ||||||||||||||||||||
| History | |||||||||||||||||||||
| Discovery | André-Marie Ampère (1810) | ||||||||||||||||||||
| First isolation | Henri Moissan (June 26, 1886) | ||||||||||||||||||||
| Named by | Humphry Davy | ||||||||||||||||||||
| Main isotopes of fluorine | |||||||||||||||||||||
|
|||||||||||||||||||||
| reference | |||||||||||||||||||||
Fluorine (symbol F) is a chemical element that is very poisonous. Its atomic number (which is the number of protons in it) is 9, and its atomic mass is 19. It is part of the Group 7 (halogens) on the periodic table of elements.
Contents
Properties
Fluorine is a light yellow diatomic gas. It is very reactive gas, which exists as diatomic molecules. It is actually the most reactive element. Fluorine has a very high attraction for electrons, because it is missing one. This makes it the most powerful oxidizing agent. It can rip electrons from water (making oxygen) and ignite propane on contact. It does not need a spark. Metals can catch on fire when placed in a stream of fluorine. After it is reduced by reacting with other things, it forms the stable fluoride ion. Fluorine is very poisonous. Fluorine bonds very strongly with carbon. It can react with the unreactive noble gases. It explodes when mixed with hydrogen. The melting point of fluorine is -363.33°F (-219.62°C), the boiling point is -306.62°F (-188.12°C).
Chemical compounds
Chemical compounds containing fluorine ions are called fluorides. Fluorine only exists in one oxidation state: -1.
- Aluminium fluoride
- Antimony trifluoride
- Antimony pentafluoride
- Arsenic trifluoride
- Arsenic pentafluoride
- Bismuth(III) fluoride
- Bismuth(V) fluoride
- Bromine trifluoride
- Bromine pentafluoride
- Chlorine monofluoride
- Chlorine trifluoride
- Cobalt(II) fluoride
- Cobalt(III) fluoride
- Disulfur decafluoride
- Hydrofluoric acid, a solution of hydrogen fluoride in water
- Hydrogen fluoride
- Iodine trifluoride
- Iodine pentafluoride
- Iodine heptafluoride
- Manganese(II) fluoride
- Manganese(III) fluoride
- Manganese(IV) fluoride
- Potassium fluoride
- Selenium tetrafluoride
- Selenium hexafluoride
- Silver(I) fluoride, brown-yellow
- Silver(II) fluoride, highly reactive, white or gray
- Sodium aluminium fluoride, cryolite
- Sodium fluoride
- Sulfur hexafluoride
- Sulfur tetrafluoride
- Tellurium(IV) fluoride
- Tellurium(VI) fluoride
- Thallium(I) fluoride
- Thallium(III) fluoride
- Tin(II) fluoride
- Tin(IV) fluoride
- Zinc fluoride
Occurrence
Fluorine is not found as an element on the earth; it is much too reactive. Several fluorides are found in the earth, though. When calcium phosphate is reacted with sulfuric acid to make phosphoric acid, some hydrofluoric acid is produced. Also, fluorite can be reacted with sulfuric acid to make hydrofluoric acid. Is a luiquid of fluoride U. Fluorine naturally occurs on the earths' crust in rocks, coal and clay.
Preparation
Fluorine is normally made by electrolysis. Hydrogen fluoride is dissolved in potassium fluoride. This mixture is melted and an electric current is passed through it. This is electrolysis. Hydrogen is produced at one side and fluorine at the other side. If the sides are not separated, the cell may explode.
Someone made fluorine in 1986 without using electrolysis. They produced manganese(IV) fluoride by using various chemical compounds, which released fluorine gas.
Uses
Fluorine is used to enrich uranium for nuclear weapons. It is also used to make sulfur hexafluoride. Sulfur hexafluoride is used to propel stuff out of an aerosol can. It is also used to make integrated circuits. Fluorine compounds have many uses. Fluoride ions are in fluorine compounds. Fluoride ions can be in toothpaste. Some are used in nonstick coatings. Freons contain fluorine.
Safety
Fluorine as an element is extremely reactive and toxic. It can react with almost everything, even glass. Fluorine is also poisonous.
Fluoride ions are somewhat toxic. If too much toothpaste containing fluoride is eaten then fluoride poisoning may occur. Fluoride is not reactive, though.
Related pages
Images for kids
-
Steelmaking illustration from De re metallica
-
Chlorine trifluoride, whose corrosive potential ignites asbestos, concrete, sand and other fire retardants
-
Immiscible layers of colored water (top) and much denser perfluoroheptane (bottom) in a beaker; a goldfish and crab cannot penetrate the boundary; quarters rest at the bottom.
-
Fluorosurfactant-treated fabrics are often hydrophobic
-
Perfluorooctanesulfonic acid, a key Scotchgard component until 2000
See also
 In Spanish: Flúor para niños
In Spanish: Flúor para niños