Enthalpy of vaporization facts for kids
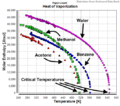
The Enthalpy of vaporization, also called the latent heat of vaporization is the change in the enthalpy needed to change an amount of liquid into a gas. It happens at a given pressure. The enthalpies of vaporization are usually measured at the normal boiling point for that substance. The units are usually J/mol.
An example is when the cooling effects of vaporization controls the body temperature of humans. Warm weather or exercise creates heat in the body. This causes the body to sweat (or perspire). The body feels cooler when the liquid sweat evaporates. As the humidity increases, the rate of evaporation decreases. This determines the body's comfort level.
Related pages
Images for kids
See also
 In Spanish: Entalpía de vaporización para niños
In Spanish: Entalpía de vaporización para niños

All content from Kiddle encyclopedia articles (including the article images and facts) can be freely used under Attribution-ShareAlike license, unless stated otherwise. Cite this article:
Enthalpy of vaporization Facts for Kids. Kiddle Encyclopedia.