Unbiunium facts for kids
Unbiunium is a hypothetical (theoretical or imagined) element of the periodic table. It is also known as eka-actinium. The atomic number of this element is 121. It has the symbol Ubu. The name Unbiunium and the symbol Ubu are temporary IUPAC names. This name and symbol exist until a permanent name is decided. It is expected to be a F-block element and an superactinides. It is the third element in the eighth period of the periodic table.
The synthesis of unbiunium was first attempted in 1977 by bombarding a target of uranium-238 with copper-65 ions at the GSI Helmholtz Centre for Heavy Ion Research in Darmstadt, Germany:
- 238U + 65Cu → 303Ubu
No atoms were identified.
| Periodic table | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
Images for kids
-
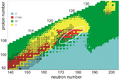
Chart of nuclide stability as used by the Dubna team in 2010. Characterized isotopes are shown with borders. Beyond element 118 (oganesson, the last known element), the line of known nuclides is expected to rapidly enter a region of instability, with no half-lives over one microsecond after element 121. The elliptical region encloses the predicted location of the island of stability.
-
Predicted decay modes of superheavy nuclei. The line of synthesized proton-rich nuclei is expected to be broken soon after Z = 120, because of the shortening half-lives until around Z = 124, the increasing contribution of spontaneous fission instead of alpha decay from Z = 122 onward until it dominates from Z = 125, and the proton drip line around Z = 130. Beyond this is a region of slightly increased stability of second-living nuclides around Z = 124 and N = 198, but it is separated from the mainland of nuclides that may be obtained with current techniques. The white ring denotes the expected location of the island of stability; the two squares outlined in white denote 291Cn and 293Cn, predicted to be the longest-lived nuclides on the island with half-lives of centuries or millennia.
See also
 In Spanish: Unbiunio para niños
In Spanish: Unbiunio para niños