Matter facts for kids
    |
|
| Matter is usually classified into three classical states, with plasma sometimes added as a fourth state. From top to bottom: quartz (solid), water (liquid), nitrogen dioxide (gas), and a plasma globe (plasma). |
Matter is the substance of which all material is made. That means objects which have mass. More specifically, they must have rest mass, which is a form of energy that matter has even when it is not moving (it has no kinetic energy), is extremely cold (it has no thermal energy), etc. Matter is a word that is sometimes used in varying ways in everyday life, whereas mass is a well-defined concept and quantity at least in physics. They are not the same thing, though they are related.
Ordinary matter is made of tiny particles called atoms. The atoms have spaces between them and they move or vibrate all the time. The particles move faster and move further apart when heated, and the reverse when cooled.
Contents
Baryonic matter
Nearly all matter that may be experienced in everyday life is baryonic matter. This includes atoms of any sort, and gives those the property of mass. Non-baryonic matter, as implied by the name, is any sort of matter that is not composed mainly of baryons. This might include neutrinos and free electrons, dark matter, such as supersymmetric particles, axions, and black holes.
The very existence of baryons is a significant issue in cosmology. It is assumed that the Big Bang produced a state with equal amounts of baryons and antibaryons. The process by which baryons came to outnumber their antiparticles is called baryogenesis.
Properties of matter
Matter can be directly experienced through the senses. It has properties which can be measured, such as mass, volume, density, and qualitative properties such as taste, smell and colour, for instance.
Examples of matter
All physical bodies in the universe are made of matter: galaxies, stars and planets, rocks, water and air. Living organisms like plants, animals and humans are also composed of matter.
In physics, the universe also contains things that aren't matter, including some elementary particles that have no rest mass. Photons (electromagnetic radiation such as light) are a familiar example.
In addition to its rest mass, matter can contain other forms of energy, which aren't matter but allow them to interact with each other by exchanging kinetic energy, heat, light, sound waves, etc.
Outside of the physical sciences, there can be many other things that aren't matter or energy. Just for example, emotions can be experienced or ideas can be had.
Composition
The structure and composition of matter is investigated by breaking matter into smaller and smaller pieces. Hence, living organisms are made up of cells. Cells are composed of molecules, which are sets of atoms bonded together. A molecule is formed when two or more atoms join together chemically. Each atom, in turn, is an assemblage of elementary particles.
States of matter
Physicists also classify matter in a few broad categories, called states, with quite different properties:
- Solids are material objects made up of molecules and atoms so strongly bonded together that they tend to keep their shape even when moved around, though they can deform under stress. Examples: a rock, a table, a knife, a block of ice.
- Fluids are amounts of matter composed of molecules and atoms weakly bonded together. They do not have a proper shape. There are two types of fluids:
- Liquids include condensed forms of matter, like solids, but where the bonds between the constituting elements (molecules, atoms) allow them to move with respect to each other while continuing to stick together in bulk: they maintain a definite surface. Liquids adopt the shape of the recipients in which they are contained. Examples: water, oil, blood, lava, soft drinks.
- Gases are amounts of matter where the bonds between the constituting elements (molecules, atoms) are so loose or weak that they can move independently from each other. Gases do not exhibit a proper surface, they tend to expand to occupy the whole volume available. Examples: air, water vapor, helium.
- Plasmas are made of ionized matter, they are mostly of interest to scientists. Examples: the Earth's ionosphere, the Sun's corona. The particles in a plasma are a mixture between a liquid and a gas. The particles are free to move, like a liquid, and the attraction is weak, like a gas. This state of matter is not fully understood. An example of plasma can be found in lightning.
- A Bose–Einstein condensate (BEC) is a state of matter of a dilute gas of bosons cooled to temperatures very near absolute zero (0 K or −273.15 °C)
A given amount of matter may change from one state to another depending on its temperature and pressure. On Earth, water can exist simultaneously in three states: solid (ice), liquid water (lakes, oceans) and gas (vapor or steam).
Related pages
Images for kids
-
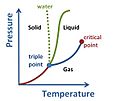
Phase diagram for a typical substance at a fixed volume. Vertical axis is Pressure, horizontal axis is Temperature. The green line marks the freezing point (above the green line is solid, below it is liquid) and the blue line the boiling point (above it is liquid and below it is gas). So, for example, at higher T, a higher P is necessary to maintain the substance in liquid phase. At the triple point the three phases; liquid, gas and solid; can coexist. Above the critical point there is no detectable difference between the phases. The dotted line shows the anomalous behavior of water: ice melts at constant temperature with increasing pressure.
-
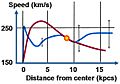
Galaxy rotation curve for the Milky Way. Vertical axis is speed of rotation about the galactic center. Horizontal axis is distance from the galactic center. The sun is marked with a yellow ball. The observed curve of speed of rotation is blue. The predicted curve based upon stellar mass and gas in the Milky Way is red. The difference is due to dark matter or perhaps a modification of the law of gravity. Scatter in observations is indicated roughly by gray bars.
See also
 In Spanish: Materia para niños
In Spanish: Materia para niños