Water (molecule) facts for kids
| Water (H2O) | |
|---|---|
  |
|
| General | |
| Systematic name | Water |
| Other names | Aqua Hydrogen oxide Hydrogen hydroxide Hydrate Oxidane Hydric acid Dihydrogen monoxide Hydrohydroxic acid μ-Oxido dihydrogen |
| Molecular formula | HOH or H2O |
| Molar mass | 18.01524 g·mol−1 |
| Appearance | transparent, almost colorless liquid with a slight hint of blue |
| CAS number | [7732-18-5] |
| see also | Water (data page) |
| Properties | |
| Density and phase | 1000 kg·m−3, liquid (4 °C) 917 kg·m−3, solid |
| Melting point | 0 °C, 32 °F (273.15 K) |
| Boiling point | 100 °C, 212 °F (373.15 K) |
| Triple point | 273.16 K, 611.73 Pa |
| Critical point | 647 K, 22.1 MPa |
| Specific heat capacity (gas) |
cp=1970 J·kg−1·K−1 @ 300 K cv=1510 J·kg−1·K−1 @ 300 K |
| Specific heat capacity (liquid) |
4186 J·kg−1·K−1 |
| Specific heat capacity (solid) |
2060 J·kg−1·K−1 |
| Acidity (pKa) | 15.74 |
| Basicity (pKb) | 15.74 |
| Viscosity | 0.001 Pa·s at 20 °C |
| Surface Tension at 20 °C | 7.28 N·m−1 |
| Structure | |
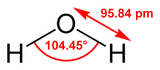
| Molecular shape | non-linear bent |
| Crystal structure | Hexagonal See ice |
| Dipole moment | 1.85 D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Drowning |
| NFPA 704 | |
| RTECS number | ZC0110000 |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Related solvents | acetone methanol |
| Related compounds | water vapor ice heavy water |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references |
|
Water (H2O, HOH) covers 70-75% of the Earth's surface in its liquid and solid (ice) states, and is present in the atmosphere as a vapor. It is the most abundant molecule on the Earth's surface.
At room temperature, it is a nearly colorless, flavorless, and odorless liquid. Many substances dissolve in water and it is commonly seen as the universal solvent; because of this, water in nature and in use is rarely clean, and may have some properties different than those in the laboratory. But there are many compounds that are essentially, if not completely, insoluble in water. Water is the only common, pure substance found naturally in all three states of matter—for other substances, see Chemical properties.
Pure water is tasteless. It is the other chemicals in the water which may give water a flavour.
Liquid water freezes and becomes solid ice at a temperature of 0° Celsius (32° Fahrenheit or 273 kelvin).
Contents
Anomalous properties of water
Generally, the volume of a liquid increases when heated, however the volume of water decreases when heated within the temperature range of 0°C to 4°C. Its volume increases only when heated above 4°C. Such behavior is one of a number of water's anomalous properties.
This property of volume decrease allows fish and other aquatic animals to survive in a body of water when ice has frozen on the surface. In cold climates, when the temperature of a body of water reaches at 4°C, the layers of water near the top in contact with cold air continue to lose heat energy and their temperature falls below 4°C. On cooling below 4°C, these layers rise rather than sink, as water has maximum density at 4°C. Thus the layer of water with a temperature of 4°C remains at the bottom, while layers of water 3°C, 2°C, 1°C and 0°C are formed above. Because ice is poor conductor of heat, it does not allow heat energy transfer from the liquid water beneath the layers of ice, which prevents the liquid water from freezing. Hence, aquatic creatures survive in such places.
Physical properties
Water is the chemical substance with chemical formula H2O; one molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom. Water is a tasteless, odorless liquid at ambient temperature and pressure. Liquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour. This can easily be observed in a water-filled bath or wash-basin whose lining is white. Large ice crystals, as in glaciers, also appear blue.
Under standard conditions, water is primarily a liquid, unlike other analogous hydrides of the oxygen family, which are generally gaseous. This unique property of water is due to hydrogen bonding. The molecules of water are constantly moving in relation to each other, and the hydrogen bonds are continually breaking and reforming at timescales faster than 200 femtoseconds (2×10−13 seconds). However, these bonds are strong enough to create many of the peculiar properties of water, some of which make it integral to life.
Water, ice, and vapor
Within the Earth's atmosphere and surface, the liquid phase is the most common and is the form that is generally denoted by the word "water". The solid phase of water is known as ice and commonly takes the structure of hard, amalgamated crystals, such as ice cubes, or loosely accumulated granular crystals, like snow. Aside from common hexagonal crystalline ice, other crystalline and amorphous phases of ice are known. The gaseous phase of water is known as water vapor (or steam). Visible steam and clouds are formed from minute droplets of water suspended in the air.
Images for kids
-
Dew drops adhering to a spider web
-
Rain water flux from a canopy. Among the forces that govern drop formation: Surface tension, Cohesion (chemistry), Van der Waals force, Plateau–Rayleigh instability.
-
This paper clip is under the water level, which has risen gently and smoothly. Surface tension prevents the clip from submerging and the water from overflowing the glass edges.
-
Presence of colloidal calcium carbonate from high concentrations of dissolved lime turns the water of Havasu Falls turquoise.
See also
 In Spanish: Agua para niños
In Spanish: Agua para niños