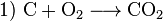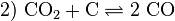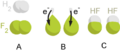Redox facts for kids

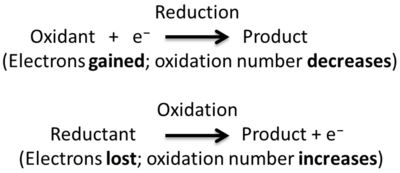
Redox (shorthand for reduction/oxidation) describes all chemical reactions in which atoms have an increase or decrease in oxidation number (oxidation state).
An oxidation number is a number assigned to an element in chemical combination that represents the number of electrons lost (or gained, if the number is negative), by an atom of that element in the compound.
The term redox comes from the two concepts of reduction and oxidation. It can be explained in simple terms:
- Oxidation describes the loss of electrons by a molecule, atom or ion
- Reduction describes the gain of electrons by a molecule, atom or ion
Whether an electron is gained or lost can be easily memorised by the abbreviation OIL RIG, which stands for, "Oxidation Is Loss," or losing electrons, and "Reduction Is Gain," or gaining electrons. Redox reactions can also happen by sharing electrons to form a product by covalent bonding.
In an oxidation reduction reaction, the cation gives an electron to the anion because both ions will have a different charge to attract each other with. In an oxidation reduction reaction, the oxidizing reagent pulls an electron from the other atom to have a net positive charge. The reducing reagent gives an electron to have a net negative charge. However, there are exceptions.
Chemical process
Redox is a chemical process. It can be described in chemical formulas. This example describes the process that occurs in a blast furnace, where iron (Fe) reacts with carbon (C):

- Now elementary iron has been processed.
Related pages
Images for kids
-
Sodium and fluorine bonding ionically to form sodium fluoride. Sodium loses its outer electron to give it a stable electron configuration, and this electron enters the fluorine atom exothermically. The oppositely charged ions are then attracted to each other. The sodium is oxidized; and the fluorine is reduced.
-
Oxides, such as iron(III) oxide or rust, which consists of hydrated iron(III) oxides Fe2O3·nH2O and iron(III) oxide-hydroxide (FeO(OH), Fe(OH)3), form when oxygen combines with other elements
-
Iron rusting in pyrite cubes
See also
 In Spanish: Reducción-oxidación para niños
In Spanish: Reducción-oxidación para niños