John Dalton facts for kids
Quick facts for kids
John Dalton
|
|
|---|---|

|
|
| Born | 6 September 1766 |
| Died | 27 July 1844 (aged 77) |
| Cause of death | Stroke |
| Nationality | British |
| Known for | Atomic theory, Law of Multiple Proportions, Dalton's Law of Partial Pressures, Daltonism |
| Awards | Royal Medal (1826) |
| Scientific career | |
| Notable students | James Prescott Joule |
| Influences | John Gough |
| Author abbrev. (botany) | Jn.Dalton |
| Signature | |
John Dalton FRS (6 September 1766 – 27 July 1844) was an English chemist, physicist, and meteorologist. He is best known for introducing the atomic theory into chemistry, and for his research into colour blindness, sometimes referred to as Daltonism in his honour. Both he and his brother were colour blind and he recognised that the condition must be hereditary.
Contents
Early life and career
John Dalton was born into a Quaker family in Eaglesfield, near Cockermouth, in Cumberland, England. He received his early education from his father and from Quaker John Fletcher, who ran a private school in the nearby village of Pardshaw Hall. Dalton's family was too poor to support him for long and he began to earn his living at the age of ten. It is said he began teaching at a local school at age 12, and became proficient in Latin at age 14.
When he was 15, Dalton joined his older brother Jonathan in running a Quaker school in Kendal, Westmorland. He acquired much scientific knowledge from informal instruction by John Gough, a blind philosopher who was gifted in the sciences and arts. At the age of 27 he was appointed teacher of mathematics and natural philosophy at the "New College" in Manchester. He remained there until the age of 34, when the college's worsening financial situation led him to resign his post and begin a new career as a private tutor in mathematics and natural philosophy.
Dalton's Atomic Theory
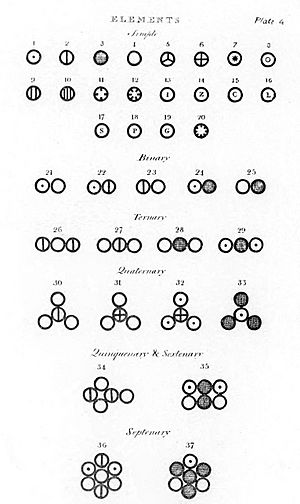
Dalton's Atomic theory is a scientific theory about atoms. Dalton made the theory to explain why elements would combine in certain ways The idea of atoms was already known at the time, but not widely accepted. Dalton's theory of atoms was based on actual observation. Before this, ideas about atoms were based more on philosophy.
The main points of Dalton's atomic theory are:
- Elements are made of extremely small particles called atoms.
- Atoms of a given element are identical in size, mass and other properties; atoms of different elements differ in size, mass and other properties.
- Atoms cannot be subdivided, created or destroyed.
- Atoms of different elements combine in simple whole-number ratios to form chemical compounds.
- In chemical reactions, atoms are combined, separated or rearranged.
Although not a part of the Dalton's original atomic theory, it is believed that Dalton's atomic theory contained the way that the atoms use to bond together. Dalton used his own symbols to visually represent the atomic structure of compounds. They were depicted in the New System of Chemical Philosophy, where he listed 20 elements and 17 simple molecules.
Personal life
Dalton never married and had only a few close friends. As a Quaker, he lived a modest and unassuming personal life.
For the 26 years prior to his death, Dalton lived in a room in the home of the Rev W. Johns, a published botanist, in George Street, Manchester.
Dalton's daily round of laboratory work and tutoring in Manchester was broken only by annual excursions to the Lake District and occasional visits to London. In 1822 he paid a short visit to Paris, where he met many distinguished resident men of science. He attended several of the earlier meetings of the British Association at York, Oxford, Dublin and Bristol.
Disability and death
Dalton suffered a minor stroke in 1837, and a second in 1838 left him with a speech impairment, although he remained able to perform experiments. In May 1844 he had another stroke; on 26 July 1844 he recorded with trembling hand his last meteorological observation. On 27 July 1844, in Manchester, Dalton fell from his bed and was found lifeless by his attendant.
Dalton was accorded a civic funeral with full honours. His body lay in state in Manchester Town Hall for four days and more than 40,000 people filed past his coffin. The funeral procession included representatives of the city’s major civic, commercial, and scientific bodies. He was buried in Manchester in Ardwick cemetery. The cemetery is now a playing field, but pictures of the original grave may be found in published materials.
Legacy

- Much of Dalton's written work, collected by the Manchester Literary and Philosophical Society, was damaged during bombing on 24 December 1940. It prompted Isaac Asimov to say, "John Dalton's records, carefully preserved for a century, were destroyed during the World War II bombing of Manchester. It is not only the living who are killed in war". The damaged papers are in the John Rylands Library.
- A bust of Dalton, by Chantrey, paid for by public subscription was placed in the entrance hall of the Royal Manchester Institution. Chantrey's large statue of Dalton, erected while Dalton was alive was placed in Manchester Town Hall in 1877. He "is probably the only scientist who got a statue in his lifetime".
- John Dalton Street connects Deansgate and Albert Square in the centre of Manchester.
- The John Dalton building at Manchester Metropolitan University is occupied by the Faculty of Science and Engineering. Outside it stands William Theed's statue of Dalton, erected in Piccadilly in 1855, and moved there in 1966 .
- A blue plaque commemorates the site of his laboratory at 36 George Street in Manchester.
- The University of Manchester established two Dalton Chemical Scholarships, two Dalton Mathematical Scholarships, and a Dalton Prize for Natural History. A hall of residence is named Dalton Hall.
- The Dalton Medal, has been awarded only twelve times by the Manchester Literary and Philosophical Society.
- A lunar crater was named after Dalton.
- "Daltonism" became a common term for colour blindness and daltonien is the French word for "colour blind".
- The inorganic section of the UK's Royal Society of Chemistry is named after Dalton (Dalton Division), and the society's academic journal for inorganic chemistry also bears his name (Dalton Transactions).
- In honour of Dalton's work, many chemists and biochemists use the (unofficial) designation dalton (abbreviated Da) to denote one atomic mass unit (1/12 the weight of a neutral atom of carbon-12).
- Quaker schools have named buildings after Dalton: for example, a school house in the primary sector of Ackworth School, is called Dalton.
- Dalton Township in southern Ontario was named after him. In 2001 the name was lost when the township was absorbed into the City of Kawartha Lakes but in 2002 the Dalton name was affixed to a new park, Dalton Digby Wildlands Provincial Park.
Images for kids
See also
 In Spanish: John Dalton para niños
In Spanish: John Dalton para niños