Beer–Lambert law facts for kids
The Beer-Lambert law is commonly applied to chemical analysis measurements to determine the concentration of chemical species that absorb light. It is often referred to as Beer's law. In physics, the Bouguer–Lambert law is an empirical law which relates the extinction or attenuation of light to the properties of the material through which the light is travelling. It had its first use in astronomical extinction. The fundamental law of extinction (the process is linear in the intensity of radiation and amount of radiatively active matter, provided that the physical state is held constant) is sometimes called the Beer-Bouguer-Lambert law or the Bouguer-Beer-Lambert law or merely the extinction law. The extinction law is also used in understanding attenuation in physical optics, for photons, neutrons, or rarefied gases. In mathematical physics, this law arises as a solution of the BGK equation.
Contents
History
Bouguer-Lambert law: This law is based on observations made by Pierre Bouguer before 1729. It is often attributed to Johann Heinrich Lambert, who cited Bouguer's Essai d'optique sur la gradation de la lumière (Claude Jombert, Paris, 1729) – and even quoted from it – in his Photometria in 1760. Lambert expressed the law, which states that the loss of light intensity when it propagates in a medium is directly proportional to intensity and path length, in the mathematical form used today.
Lambert began by assuming that the intensity I of light traveling into an absorbing body would be given by the differential equation: 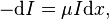 which is compatible with Bouguer's observations. The constant of proportionality μ was often termed the "optical density" of the body. Integrating to find the intensity at a distance d into the body, one obtains:
which is compatible with Bouguer's observations. The constant of proportionality μ was often termed the "optical density" of the body. Integrating to find the intensity at a distance d into the body, one obtains:  For a homogeneous medium, this reduces to:
For a homogeneous medium, this reduces to:  from which follows the exponential attenuation law:
from which follows the exponential attenuation law: 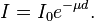
Beer's law: Much later, in 1852, the German scientist August Beer studied another attenuation relation. In the introduction to his classic paper, he wrote: "The absorption of light during the irradiation of a colored substance has often been the object of experiment; but attention has always been directed to the relative diminution of the various colors or, in the case of crystalline bodies, the relation between the absorption and the direction of polarization. Concerning the absolute magnitude of the absorption that a particular ray of light suffers during its propagation through an absorbing medium, there is no information available." By studying absorption of red light in colored aqueous solutions of various salts, he concluded that "the transmittance of a concentrated solution can be derived from a measurement of the transmittance of a dilute solution". It is clear that he understood the exponential relationship, as he wrote: "If 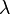 is the coefficient (fraction) of diminution, then this coefficient (fraction) will have the value
is the coefficient (fraction) of diminution, then this coefficient (fraction) will have the value 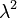 for double this thickness." Furthermore Beer stated: "We shall take the absorption coefficient to be the coefficient giving the diminution in amplitude suffered by a light ray as it passes through a unit length of an absorbing material. We then have, according to theory, and as I have found verified by experiment,
for double this thickness." Furthermore Beer stated: "We shall take the absorption coefficient to be the coefficient giving the diminution in amplitude suffered by a light ray as it passes through a unit length of an absorbing material. We then have, according to theory, and as I have found verified by experiment, 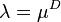 where
where 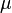 is the absorption coefficient and D the length of the absorbing material traversed in the experiment." This is the relationship that might properly be called Beer's law. There is no evidence that Beer saw concentration and path length as symmetrical variables in an equation in the manner of the Beer-Lambert law.
is the absorption coefficient and D the length of the absorbing material traversed in the experiment." This is the relationship that might properly be called Beer's law. There is no evidence that Beer saw concentration and path length as symmetrical variables in an equation in the manner of the Beer-Lambert law.
Beer-Lambert law: The modern formulation of the Beer–Lambert law combines the observations of Bouguer and Beer into the mathematical form of Lambert. It correlates the absorbance, most often expressed as the negative decadic logarithm of the transmittance, to both the concentrations of the attenuating species and the thickness of the material sample. An early, possibly the first, modern formulation was given by Robert Luther and Andreas Nikolopulos in 1913.
Differences between Bouguer and Beer in application areas
While the observations of Bouguer and Beer have a similar form in the Beer-Lambert law, their areas of observation were very different. For both experimenters, the incident beam was well collimated, with a light sensor which preferentially detected directly transmitted light.
Beer specifically looked at solutions. Solutions are homogeneous and do not scatter light (Ultraviolet, visible, Infrared) of wavelengths commonly used in analytical spectroscopy (except upon entry and exit). The attenuation of a beam of light within a solution is assumed to be only due to absorption. In order to approximate the conditions required for the Beer Lambert law to hold, often the intensity of transmitted light through a reference sample 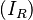 consisting of pure solvent is measured, and compared to the intensity of light transmitted through a sample
consisting of pure solvent is measured, and compared to the intensity of light transmitted through a sample 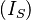 , with the absorbance of the sample taken as:
, with the absorbance of the sample taken as: 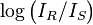 . It is for this case that the common mathematical formulation (see below) applies:
. It is for this case that the common mathematical formulation (see below) applies: 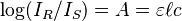
Bouguer looked at astronomical phenomena where the size of a detector is very small compared to the distance traveled by the light. In this case, any light that is scattered by a particle, either in the forward or backward direction, will not strike the detector. The loss of intensity to the detector will be due to both absorption and scatter. Consequently, the total loss is called attenuation (rather than absorption). A single measurement cannot separate the two, but conceptually the contribution of each can be separated in the attenuation coefficient. If 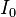 is the intensity of the light at the beginning of the travel and
is the intensity of the light at the beginning of the travel and 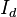 is the intensity of the light detected after travel of a distance
is the intensity of the light detected after travel of a distance 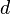 , the fraction transmitted,
, the fraction transmitted, 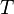 , is given by:
, is given by:  , where
, where 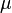 is called an attenuation constant or coefficient. The amount of light transmitted is falling off exponentially with distance. Taking the natural logarithm in the above equation, we get:
is called an attenuation constant or coefficient. The amount of light transmitted is falling off exponentially with distance. Taking the natural logarithm in the above equation, we get: 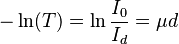 . For scattering media, the constant is often divided into two parts,
. For scattering media, the constant is often divided into two parts, 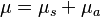 , separating it into a scattering coefficient,
, separating it into a scattering coefficient, 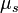 , and an absorption coefficient,
, and an absorption coefficient, 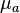 .
.
Absorptivity, Cross Sections and Units of Coefficients
The fundamental law of extinction states that the extinction process is linear in the intensity of radiation and amount of radiatively active matter, provided that the physical state is held constant. (Neither concentration or length are fundamental parameters.) There are two factors that determine the degree to which a medium containing particles will attenuate a light beam: the number of particles encountered by the light beam, and the degree to which each particle extinguishes the light.
For the case of absorption (Beer), this later quantity is called the absorptivity [ ], which is defined as "the property of a body that determines the fraction of incident radiation absorbed by the body". The Beer-Lambert law
], which is defined as "the property of a body that determines the fraction of incident radiation absorbed by the body". The Beer-Lambert law ![[\log (I_0/I)= A= \epsilon \ell c ]](/images/math/b/2/4/b24df0088e1413e5307e9d719ad6772a.png) uses concentration and length in order to determine the number of particles the beam encounters. If we know the area of a collimated beam (directed radiation), we can get the number of particles in a distance. The number of particles encountered can be calculated from Avagadro's number, the molar concentration, the cross-sectional area of the incident beam
uses concentration and length in order to determine the number of particles the beam encounters. If we know the area of a collimated beam (directed radiation), we can get the number of particles in a distance. The number of particles encountered can be calculated from Avagadro's number, the molar concentration, the cross-sectional area of the incident beam ![[(molecules/mol) (mol/L) (1 L /1000cm^3)(cm^2)= molecules/cm]](/images/math/3/5/f/35f336c61459362ae2426a603284842c.png) .
.
There must be a large number of particles that are uniformly distributed for this relationship to hold. In practice, the beam area is thought of as a constant, and since the fraction [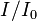 ] has the area in both the numerator and denominator, the beam area cancels in the calculation of the absorbance. The units of the absorptivity must match the units in which the sample is described. For example, if the sample is described by mass concentration (g/L) and length (cm), then the units on the absorptivity would be [ L g−1 cm−1], so that the absorbance has no units.
] has the area in both the numerator and denominator, the beam area cancels in the calculation of the absorbance. The units of the absorptivity must match the units in which the sample is described. For example, if the sample is described by mass concentration (g/L) and length (cm), then the units on the absorptivity would be [ L g−1 cm−1], so that the absorbance has no units.
For the case of "extinction" (Bouguer), the sum of absorption and scatter, the terms absorption, scattering, and extinction cross-sections are often used. The fraction of light extinguished by the sample may be described by the extinction cross section (fraction extinguished per particle). the number of particles in a unit distance and the distance in those units. For example: [ (fraction extinguished / particle) (# particles / meter) (# meters / sample) = fraction extinguished / sample ]
Mathematical formulations
A common and practical expression of the Beer–Lambert law relates the optical attenuation of a physical material containing a single attenuating species of uniform concentration to the optical path length through the sample and absorptivity of the species. This expression is: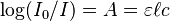 where
where
- A is the absorbance
- ε is the molar attenuation coefficient or absorptivity of the attenuating species
- ℓ is the optical path length
- c is the concentration of the attenuating species
A more general form of the Beer–Lambert law states that, for N attenuating species in the material sample,![{\displaystyle \begin{align}
T &= \exp\left(-\sum_{i = 1}^N \sigma_i \int_0^\ell n_i(z)\mathrm{d}z \right) \\[4pt]
&= 10^{\;\!\wedge} \!\! \left(-\sum_{i = 1}^N \varepsilon_i \int_0^\ell c_i(z)\mathrm{d}z \right),
\end{align}}](/images/math/1/3/d/13d9bd2fcba57a93132fcae1de069907.png) or equivalently that
or equivalently that![{\displaystyle \begin{align}
\tau &= \sum_{i = 1}^N \tau_i = \sum_{i = 1}^N \sigma_i \int_0^\ell n_i(z)\,\mathrm{d}z, \\[4pt]
A &= \sum_{i = 1}^N A_i = \sum_{i = 1}^N \varepsilon_i \int_0^\ell c_i(z)\,\mathrm{d}z,
\end{align}}](/images/math/c/c/c/ccc3ccb20a4a92eb5e1888aff513d753.png) where
where
- σi is the attenuation cross section of the attenuating species i in the material sample;
- ni is the number density of the attenuating species i in the material sample;
- εi is the molar attenuation coefficient or absorptivity of the attenuating species i in the material sample;
- ci is the amount concentration of the attenuating species i in the material sample;
- ℓ is the path length of the beam of light through the material sample.
In the above equations, the transmittance T of material sample is related to its optical depth τ and to its absorbance A by the following definition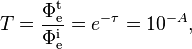 where
where
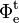 is the radiant flux transmitted by that material sample;
is the radiant flux transmitted by that material sample;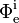 is the radiant flux received by that material sample.
is the radiant flux received by that material sample.
Attenuation cross section and molar attenuation coefficient are related by and number density and amount concentration by
and number density and amount concentration by![{\displaystyle \begin{align}
\tau &= \ell\sum_{i = 1}^N \sigma_i n_i, \\[4pt]
A &= \ell\sum_{i = 1}^N \varepsilon_i c_i.
\end{align}}](/images/math/4/4/c/44c20da9b8636a60b549a5b4b711c2b3.png)
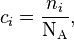 where NA is the Avogadro constant. In case of uniform attenuation, these relations become
where NA is the Avogadro constant. In case of uniform attenuation, these relations become![{\displaystyle \begin{align}
T &= \exp\left(-\ell\sum_{i = 1}^N \sigma_i n_i \right) \\[4pt]
&= 10^{\;\!\wedge} \!\! \left(-\ell\sum_{i = 1}^N \varepsilon_i c_i \right),
\end{align}}](/images/math/c/3/b/c3b2ad2bcf1778087ad14ea03bf18972.png) or equivalently
or equivalently![{\displaystyle \begin{align}
\tau &= \ell\sum_{i = 1}^N \sigma_i n_i, \\[4pt]
A &= \ell\sum_{i = 1}^N \varepsilon_i c_i.
\end{align}}](/images/math/4/4/c/44c20da9b8636a60b549a5b4b711c2b3.png)
![{\displaystyle \begin{align}
\tau &= \ell\sum_{i = 1}^N \sigma_i n_i, \\[4pt]
A &= \ell\sum_{i = 1}^N \varepsilon_i c_i.
\end{align}}](/images/math/4/4/c/44c20da9b8636a60b549a5b4b711c2b3.png) Cases of non-uniform attenuation occur in atmospheric science applications and radiation shielding theory for instance.
Cases of non-uniform attenuation occur in atmospheric science applications and radiation shielding theory for instance.
The law tends to break down at very high concentrations, especially if the material is highly scattering. Absorbance within range of 0.2 to 0.5 is ideal to maintain linearity in the Beer–Lambert law. If the radiation is especially intense, nonlinear optical processes can also cause variances. The main reason, however, is that the concentration dependence is in general non-linear and Beer's law is valid only under certain conditions as shown by derivation below. For strong oscillators and at high concentrations the deviations are stronger. If the molecules are closer to each other interactions can set in. These interactions can be roughly divided into physical and chemical interactions. Physical interaction do not alter the polarizability of the molecules as long as the interaction is not so strong that light and molecular quantum state intermix (strong coupling), but cause the attenuation cross sections to be non-additive via electromagnetic coupling. Chemical interactions in contrast change the polarizability and thus absorption.
Expression with attenuation coefficient
The law can be expressed in terms of attenuation coefficient, but in this case is better called the Bouguer-Lambert's law. The (Napierian) attenuation coefficient 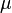 and the decadic attenuation coefficient
and the decadic attenuation coefficient  of a material sample are related to its number densities and amount concentrations as
of a material sample are related to its number densities and amount concentrations as![{\displaystyle \begin{align}
\mu(z) &= \sum_{i = 1}^N \mu_i(z) = \sum_{i = 1}^N \sigma_i n_i(z), \\[4pt]
\mu_{10}(z) &= \sum_{i = 1}^N \mu_{10,i}(z) = \sum_{i = 1}^N \varepsilon_i c_i(z)
\end{align}}](/images/math/a/c/a/aca0360d6ce866554c5607a6879f80c2.png) respectively, by definition of attenuation cross section and molar attenuation coefficient. Then the aw becomes
respectively, by definition of attenuation cross section and molar attenuation coefficient. Then the aw becomes![{\displaystyle \begin{align}
T &= \exp\left(-\int_0^\ell \mu(z)\mathrm{d}z \right) \\[4pt]
&= 10^{\;\!\wedge} \!\! \left(-\int_0^\ell \mu_{10}(z)\mathrm{d}z \right),
\end{align}}](/images/math/b/7/8/b78d47d5acef72974ecb9c6821d8a6a6.png) and
and![{\displaystyle \begin{align}
\tau &= \int_0^\ell \mu(z)\,\mathrm{d}z, \\[4pt]
A &= \int_0^\ell \mu_{10}(z)\,\mathrm{d}z.
\end{align}}](/images/math/4/9/2/492c786fe112869e96c589303086a027.png) In case of uniform attenuation, these relations become
In case of uniform attenuation, these relations become or equivalently
or equivalently![{\displaystyle \begin{align}
\tau &= \mu\ell, \\[4pt]
A &= \mu_{10}\ell.
\end{align}}](/images/math/3/3/0/33003c279e677064697260238a924b9a.png)
In many cases, the attenuation coefficient does not vary with 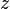 , in which case one does not have to perform an integral and can express the law as:
, in which case one does not have to perform an integral and can express the law as: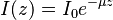 where the attenuation is usually an addition of absorption coefficient
where the attenuation is usually an addition of absorption coefficient 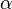 (creation of electron-hole pairs) or scattering (for example Rayleigh scattering if the scattering centers are much smaller than the incident wavelength). Also note that for some systems we can put
(creation of electron-hole pairs) or scattering (for example Rayleigh scattering if the scattering centers are much smaller than the incident wavelength). Also note that for some systems we can put 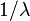 (1 over inelastic mean free path) in place of
(1 over inelastic mean free path) in place of 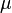 .
.
Derivation
Assume that a beam of light enters a material sample. Define z as an axis parallel to the direction of the beam. Divide the material sample into thin slices, perpendicular to the beam of light, with thickness dz sufficiently small that one particle in a slice cannot obscure another particle in the same slice when viewed along the z direction. The radiant flux of the light that emerges from a slice is reduced, compared to that of the light that entered, by 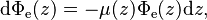 where μ is the (Napierian) attenuation coefficient, which yields the following first-order linear, ordinary differential equation:
where μ is the (Napierian) attenuation coefficient, which yields the following first-order linear, ordinary differential equation: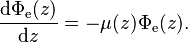 The attenuation is caused by the photons that did not make it to the other side of the slice because of scattering or absorption. The solution to this differential equation is obtained by multiplying the integrating factor
The attenuation is caused by the photons that did not make it to the other side of the slice because of scattering or absorption. The solution to this differential equation is obtained by multiplying the integrating factor throughout to obtain
throughout to obtain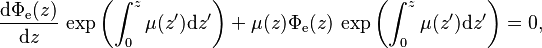 which simplifies due to the product rule (applied backwards) to
which simplifies due to the product rule (applied backwards) to![{\displaystyle \frac{\mathrm{d}}{\mathrm{d}z}\left[\Phi_\mathrm{e}(z) \exp\left(\int_0^z \mu(z')\mathrm{d}z' \right)\right] = 0.}](/images/math/7/7/1/771742ce4d425eb78052820501d1a79f.png)
Integrating both sides and solving for Φe for a material of real thickness ℓ, with the incident radiant flux upon the slice 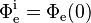 and the transmitted radiant flux
and the transmitted radiant flux 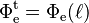 gives
gives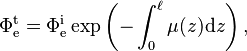 and finally
and finally
Since the decadic attenuation coefficient μ10 is related to the (Napierian) attenuation coefficient by 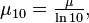 we also have
we also have![{\displaystyle \begin{align}
T &= \exp\left(-\int_0^\ell \ln{10}\,\mu_{10}(z)\mathrm{d}z \right) \\[4pt]
&= 10^{\;\!\wedge} \!\! \left( -\int_0^\ell \mu_{10}(z)\mathrm{d}z \right).
\end{align}}](/images/math/e/c/a/eca841cebc65496881f60b6a1e929b67.png)
To describe the attenuation coefficient in a way independent of the number densities ni of the N attenuating species of the material sample, one introduces the attenuation cross section 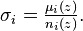 σi has the dimension of an area; it expresses the likelihood of interaction between the particles of the beam and the particles of the species i in the material sample:
σi has the dimension of an area; it expresses the likelihood of interaction between the particles of the beam and the particles of the species i in the material sample: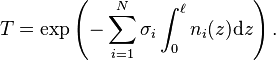
One can also use the molar attenuation coefficients 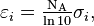 where NA is the Avogadro constant, to describe the attenuation coefficient in a way independent of the amount concentrations
where NA is the Avogadro constant, to describe the attenuation coefficient in a way independent of the amount concentrations  of the attenuating species of the material sample:
of the attenuating species of the material sample:![{\displaystyle \begin{align}
T &= \exp\left(-\sum_{i = 1}^N \frac{\ln{10}}{\mathrm{N_A}}\varepsilon_i \int_0^\ell n_i(z)\mathrm{d}z \right) \\[4pt]
&= \exp\left(-\sum_{i = 1}^N \varepsilon_i \int_0^\ell \frac{n_i(z)}{\mathrm{N_A}}\mathrm{d}z\right)^{\ln{10}} \\[4pt]
&= 10^{\;\!\wedge} \!\! \left(-\sum_{i = 1}^N \varepsilon_i \int_0^\ell c_i(z)\mathrm{d}z \right).
\end{align} }](/images/math/7/0/b/70b67cf995501130c5bf53e711492706.png)
Validity
Under certain conditions the Beer–Lambert law fails to maintain a linear relationship between attenuation and concentration of analyte. These deviations are classified into three categories:
- Real—fundamental deviations due to the limitations of the law itself.
- Chemical—deviations observed due to specific chemical species of the sample which is being analyzed.
- Instrument—deviations which occur due to how the attenuation measurements are made.
There are at least six conditions that need to be fulfilled in order for the Beer–Lambert law to be valid. These are:
- The attenuators must act independently of each other.
- The attenuating medium must be homogeneous in the interaction volume.
- The attenuating medium must not scatter the radiation—no turbidity—unless this is accounted for as in DOAS.
- The incident radiation must consist of parallel rays, each traversing the same length in the absorbing medium.
- The incident radiation should preferably be monochromatic, or have at least a width that is narrower than that of the attenuating transition. Otherwise a spectrometer as detector for the power is needed instead of a photodiode which cannot discriminate between wavelengths.
- The incident flux must not influence the atoms or molecules; it should only act as a non-invasive probe of the species under study. In particular, this implies that the light should not cause optical saturation or optical pumping, since such effects will deplete the lower level and possibly give rise to stimulated emission.
If any of these conditions are not fulfilled, there will be deviations from the Beer–Lambert law.
Application for the atmosphere
The Bouguer-Lambert law may be applied to describe the attenuation of solar or stellar radiation as it travels through the atmosphere. In this case, there is scattering of radiation as well as absorption. The optical depth for a slant path is τ′ = mτ, where τ refers to a vertical path, m is called the relative airmass, and for a plane-parallel atmosphere it is determined as m = sec θ where θ is the zenith angle corresponding to the given path. The Bouguer-Lambert law for the atmosphere is usually written 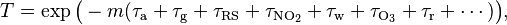 where each τx is the optical depth whose subscript identifies the source of the absorption or scattering it describes:
where each τx is the optical depth whose subscript identifies the source of the absorption or scattering it describes:
- a refers to aerosols (that absorb and scatter);
- g are uniformly mixed gases (mainly carbon dioxide (CO2) and molecular oxygen (O2) which only absorb);
- NO
2 is nitrogen dioxide, mainly due to urban pollution (absorption only); - RS are effects due to Raman scattering in the atmosphere;
- w is water vapour absorption;
- O
3 is ozone (absorption only); - r is Rayleigh scattering from molecular oxygen (O
2) and nitrogen (N
2) (responsible for the blue color of the sky); - the selection of the attenuators which have to be considered depends on the wavelength range and can include various other compounds. This can include tetraoxygen, HONO, formaldehyde, glyoxal, a series of halogen radicals and others.
m is the optical mass or airmass factor, a term approximately equal (for small and moderate values of θ) to 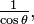 where θ is the observed object's zenith angle (the angle measured from the direction perpendicular to the Earth's surface at the observation site). This equation can be used to retrieve τa, the aerosol optical thickness, which is necessary for the correction of satellite images and also important in accounting for the role of aerosols in climate.
where θ is the observed object's zenith angle (the angle measured from the direction perpendicular to the Earth's surface at the observation site). This equation can be used to retrieve τa, the aerosol optical thickness, which is necessary for the correction of satellite images and also important in accounting for the role of aerosols in climate.
See also
 In Spanish: Ley de Beer-Lambert para niños
In Spanish: Ley de Beer-Lambert para niños
- Applied spectroscopy
- Atomic absorption spectroscopy
- Absorption spectroscopy
- Cavity ring-down spectroscopy
- Clausius-Mossotti relation
- Infra-red spectroscopy
- Job plot
- Laser absorption spectrometry
- Lorentz-Lorenz relation
- Logarithm
- Polymer degradation
- Scientific laws named after people
- Quantification of nucleic acids
- Tunable diode laser absorption spectroscopy
- Transmittance#Beer–Lambert law

