Allotropes of carbon facts for kids
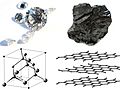
Graphite is one of the most common allotropes of carbon. Unlike diamond, graphite is a conductor, a semi-metal (an element that is partially a metal and a nonmetal, such as black phosphorus and grey selenium). It can be used, for instance, as the material in the electrodes of an electrical arc lamp. Graphite is the most stable form of solid carbon ever discovered.
Allotropes are different forms of the same chemical element. All elements are made up uniquely of their own atoms. Any physical differences are because of how the atoms are joined together. Many elements show allotropy because there are a number of ways in which the atoms can be linked together. Also there are different ways in how the molecules can be arranged to make larger structures.
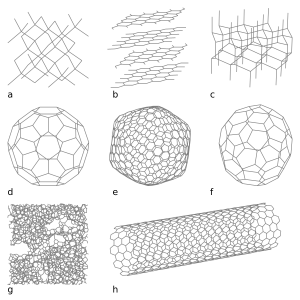
This illustration depicts eight of the allotropes (different molecular configurations) that pure carbon can take: a) Diamond b) Graphite c) Lonsdaleite d) C60 Buckminsterfullerene e) C540 see Fullerene f) C70 see Fullerene g) Amorphous carbon h) single-walled carbon nanotube
Diamond is one of the best known allotropes of carbon. Its hardness and high dispersion of light make it good for use in jewellery. It is also has industrial uses. Diamond is the hardest known natural mineral. This makes it an excellent abrasive. A diamond also holds its luster well. Both diamond and graphite have extremely high melting point, which is unusual for nonmetallic element.
Images for kids
See also
 In Spanish: Alótropos del carbono para niños
In Spanish: Alótropos del carbono para niños