Zinc oxide facts for kids
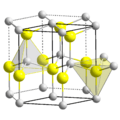
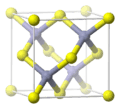
Zinc oxide is a chemical compound. Its chemical formula is ZnO. It contains zinc and oxide ions.
Properties
Zinc oxide is a white solid. It does not dissolve in water. It reacts with acids to make zinc salts. It can react with water to make zinc hydroxide. It reacts with phosphoric acid to make zinc phosphate, a cement. It reacts with hydrogen sulfide to make zinc sulfide. It dissolves in strong bases. It can be reduced by carbon to zinc metal.
Preparation
Zinc oxide can be made by heating powdered zinc metal. It can also be made by heating zinc hydroxide. It can be made by boiling zinc metal in air. Zinc carbonate makes zinc oxide when heated.
Uses
Zinc oxide is used in the vulcanization of rubber, preventing the rubber from becoming brittle. It also protects the rubber from fungi and ultraviolet light.
Zinc oxide can be added to concrete to help the making and hardening of concrete. Zinc oxide is used in calamine. It can be used as a deodorant and an antibacterial cream. Zinc oxide can be used as a sunscreen.
It can be added to food to add zinc to the diet. It can also be used in paint as a pigment. It also clings to certain metals better.
Safety
Zinc oxide is not toxic, but fine dust of zinc oxide can irritate the lungs when inhaled or aspirated.
Related pages
Images for kids
See also
 In Spanish: Óxido de zinc para niños
In Spanish: Óxido de zinc para niños