Ocean acidification facts for kids
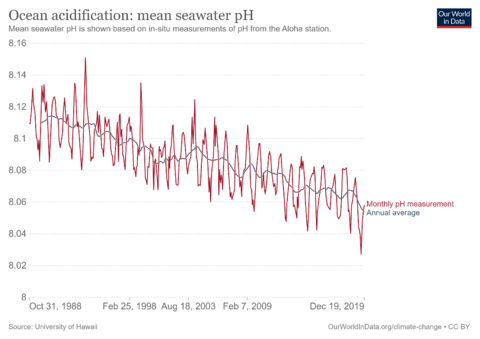
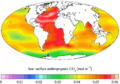
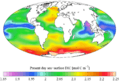
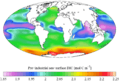
Ocean acidification is the reduction in the pH value of the Earth’s ocean. Between 1751 and 2021, the average pH value of the ocean surface has decreased from approximately 8.25 to 8.14. That means that the waters of the ocean have become more acidic.
Cause
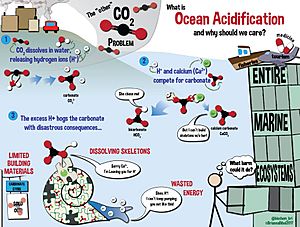
The main cause of ocean acidification is carbon dioxide emissions from human activities. The oceans absorb CO2 from the atmosphere. This leads to the formation of carbonic acid (H2CO3). It dissolves, breaking into a bicarbonate ion (HCO−
3) and a hydrogen ion (H+). The free hydrogen ions (H+) decrease the pH of the ocean, therefore increasing acidity.
As pH decreases, the concentration of carbonate ions is also decreasing. Carbonate ions are the main building block for shells and skeletons of many Marine organisms, like mollusks, oysters and corals.
Other factors that affect the atmosphere-ocean CO2 exchange and impact local ocean acidification, are: ocean currents, proximity to large continental rivers, sea ice coverage, and atmospheric exchange with nitrogen and sulfur from fossil fuel burning and agriculture.
Potential risks
Decreased ocean pH can kill many marine organisms and destroy marine ecosystems that provide food, livelihoods, and other ecosystem services for a large portion of humanity. Some 1 billion people are wholly or partially dependent on the fishing, tourism, and coastal management services provided by coral reefs. Ongoing acidification of the oceans may therefore threaten future food chains linked with the oceans.
The United Nations Sustainable Development Goal 14 ("Life below Water") has a target to "minimize and address the impacts of ocean acidification".
How to stop ocean acidification
Seawater is not acidic yet. However, if humans continue to emit greenhouse gases into the atmosphere, ocean life will be in severe danger. The only solution is to reduce carbon dioxide emissions. This can reverse the situation.
Ocean acidification has occurred previously in Earth's history. The resulting ecological collapse in the oceans had long-lasting effects on the climate.
Images for kids
-
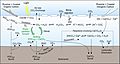
NOAA provides evidence for the upwelling of "acidified" water onto the Continental Shelf. In the figure above, note the vertical sections of (A) temperature, (B) aragonite saturation, (C) pH, (D) DIC, and (E) pCO2 on transect line 5 off Pt. St. George, California. The potential density surfaces are superimposed on the temperature section. The 26.2 potential density surface delineates the location of the first instance in which the undersaturated water is upwelled from depths of 150 to 200 m onto the shelf and outcropping at the surface near the coast. The red dots represent sample locations.
-
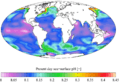
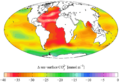
This map shows changes in the aragonite saturation level of ocean surface waters between the 1880s and the most recent decade (2006–2015). Aragonite is a form of calcium carbonate that many marine animals use to build their skeletons and shells. The lower the saturation level, the more difficult it is for organisms to build and maintain their skeletons and shells. A negative change represents a decrease in saturation.
-
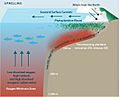
Drivers of hypoxia and ocean acidification intensification in upwelling shelf systems. Equatorward winds drive the upwelling of low dissolved oxygen (DO), high nutrient, and high dissolved inorganic carbon (DIC) water from above the oxygen minimum zone. Cross-shelf gradients in productivity and bottom water residence times drive the strength of DO (DIC) decrease (increase) as water transits across a productive continental shelf.
-
A NOAA (AOML) in situ CO2 concentration sensor (SAMI-CO2), attached to a Coral Reef Early Warning System station, utilized in conducting ocean acidification studies near coral reef areas
-
A NOAA (PMEL) moored autonomous CO2 buoy used for measuring CO2 concentration and ocean acidification studies
See also
 In Spanish: Acidificación del océano para niños
In Spanish: Acidificación del océano para niños