Intermolecular force facts for kids
In physics, chemistry, and biology, intermolecular forces are forces that act between stable molecules or between functional groups of macromolecules.
These forces are generally much weaker than the chemical bonding forces. Their bonding energies are less than a few kcal/mol. But they are responsible for many different physical, chemical, and biological phenomena. In general one distinguishes short and long range intermolecular forces. (The molecule is a substance are held together by the forces acting between the molecules which are called inter-molecular forces)
Hydrogen bonding
Hydrogen bonding is an intermolecular interaction with a hydrogen atom being present in the intermolecular bond. 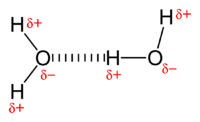
Related pages
Software for calculation of intermolecular forces
See also
 In Spanish: Fuerza intermolecular para niños
In Spanish: Fuerza intermolecular para niños

All content from Kiddle encyclopedia articles (including the article images and facts) can be freely used under Attribution-ShareAlike license, unless stated otherwise. Cite this article:
Intermolecular force Facts for Kids. Kiddle Encyclopedia.