Inner transition metal facts for kids
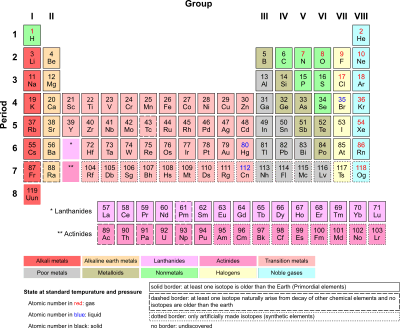
Inner transition metals (ITM) are chemical elements on the periodic table. They are normally shown in two rows below all the other elements. They include elements 57-71, or lanthanides, and 89-103, or actinides. The lanthanides are very similar, and the actinides are all radioactive.
ITMs have three incomplete outermost electron shells and are all metals. In some cases they are quite malleable and ductile. Many lanthanides such as Lutetium are used in the lighting industry. Actinides don't occur in nature (except for thorium and uranium), and are highly unstable. These elements belong to f-block and are known as the anti-penultimate shell. Its general configuration is (n-2)f^0-14(n-1)d^0-2np^6ns^2 where n= 6-7.

All content from Kiddle encyclopedia articles (including the article images and facts) can be freely used under Attribution-ShareAlike license, unless stated otherwise. Cite this article:
Inner transition metal Facts for Kids. Kiddle Encyclopedia.