Hydrogen telluride facts for kids
Quick facts for kids Hydrogen telluride |
|
|---|---|
| IUPAC name | hydrogen telluride |
| Other names | hydrotelluric acid tellane tellurium hydride |
| Identifiers | |
| CAS number | |
| PubChem | |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | colourless gas |
| Density | 3.310 g/cm3, gas 2.57 g/cm3 (-20 °C, liquid) |
| Melting point | |
| Boiling point | |
| 0.70 g/100 mL | |
| Acidity (pKa) | 2.6 |
| Structure | |
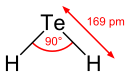
| Molecular shape | bent |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
0.7684 kJ/g |
| Hazards | |
| Main hazards | toxic |
| Related compounds | |
| Other anions | H2O H2S H2Se H2Po |
| Other cations | Na2Te Ag2Te |
| Related compounds | telluric acid tellurous acid |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Hydrogen telluride, also known as hydrotelluric acid, tellane, or tellurium hydride, is a chemical compound. It is also an acid. Its chemical formula is H2Te. It contains hydrogen and telluride ions.
Contents
Properties
Hydrogen telluride is a toxic reactive gas. It easily decomposes to hydrogen and tellurium. It also burns in air to make tellurium dioxide and water. It has a very bad smell of decayed garlic. It is almost as acidic as phosphoric acid. It reacts with some metals and metal oxides to make tellurides.
Preparation
It is made by adding a strong acid to a telluride, such as sodium telluride.
Uses
Hydrogen telluride does not have many uses because it is very toxic and unstable.
Related pages
- Aluminium telluride
- Sodium tellurite
See also
 In Spanish: Ácido telurhídrico para niños
In Spanish: Ácido telurhídrico para niños

All content from Kiddle encyclopedia articles (including the article images and facts) can be freely used under Attribution-ShareAlike license, unless stated otherwise. Cite this article:
Hydrogen telluride Facts for Kids. Kiddle Encyclopedia.