Desalination facts for kids
Desalination means any process that removes the excess salt and other minerals from water in order to obtain fresh water suitable for animal consumption or irrigation.
If almost all of the salt is removed for human consumption, sometimes the process produces table salt as a by-product.
Desalination of brackish water is done in the United States in order to meet treaty obligations for river water entering Mexico. Several Middle Eastern countries have energy reserves so great that they use desalinated water for agriculture. Saudi Arabia's desalination plants account for about 24% of total world capacity. The world's largest desalination plant is the Jebel Ali Desalination Plant (Phase 2) in UAE. It uses multi-stage flash distillation, dual-purpose and it is capable of producing 300 million cubic meters of water per year.
There are now about 21,000 desalination plants in operation around the globe. The biggest ones are in the United Arab Emirates, Saudi Arabia, and Israel. The world's largest desalination plant is located in Saudi Arabia (Ras Al-Khair Power and Desalination Plant) with a capacity of 1,401,000 cubic meters per day.
Contents
History
Desalination has been known to history for millennia. The ancient Greek philosopher Aristotle observed that "salt water, when it turns into vapour, becomes sweet and the vapour does not form salt water again when it condenses." There are numerous examples of experimentation in desalination throughout Antiquity and the Middle Ages, but desalination was never feasible on a large scale until the modern era.
The first major land-based desalination plant may have been installed under emergency conditions on an island off the coast of Tunisia in 1560. It is believed that a garrison of 700 Spanish soldiers was besieged by a large number of Turks and that, during the siege, the captain in charge fabricated a still capable of producing 40 barrels of fresh water per day, though details of the device have not been reported.
Before the Industrial Revolution, desalination was primarily of concern to oceangoing ships, which otherwise needed to keep on board supplies of fresh water.
Beginning about 1800, things started changing very rapidly as consequence of the appearance of the steam engine and the so-called age of steam. The development of a knowledge of the thermodynamics of steam processes and the need for a pure water source for its use in boilers, generated a positive effect regarding distilling systems. Additionally, the spread of European colonialism induced a need for freshwater in remote parts of the world, thus creating the appropriate climate for water desalination.
Significant research into improved desalination methods occurred in the United States after World War II. The Office of Saline Water was created in the United States Department of the Interior in 1955 in accordance with the Saline Water Conversion Act of 1952. It was merged into the Office of Water Resources Research in 1974.
The first industrial desalination plant in the United States opened in Freeport, Texas in 1961 with the hope of bringing water security to the region after a decade of drought. Vice-president Lyndon B. Johnson attended the plant's opening on June 21, 1961. President John F. Kennedy recorded a speech from the White House, describing desalination as "a work that in many ways is more important than any other scientific enterprise in which this country is now engaged."
The first commercial reverse osmosis desalination plant, Coalinga desalination plant, was inaugurated in California in 1965 for brackish water. A few years later, in 1975, the first sea water reverse osmosis desalination plant came into operation.
Applications
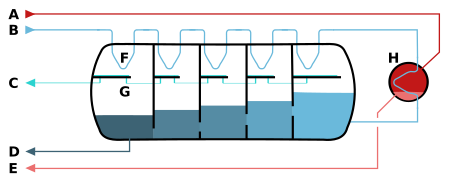
A – steam in B – seawater in C – potable water out
D – brine out (waste) E – condensate out F – heat exchange G – condensation collection (desalinated water)
H – brine heater
The pressure vessel acts as a countercurrent heat exchanger. A vacuum pump lowers the pressure in the vessel to facilitate the evaporation of the heated seawater (brine) which enters the vessel from the right side (darker shades indicate lower temperature). The steam condenses on the pipes on top of the vessel in which the fresh sea water moves from the left to the right.
Desalination may help solve the water supply problem for some water-stress regions. However, for areas that are far from the sea, like New Delhi, or in high places, like Mexico City, it may be more economical to transport fresh water from somewhere else than to desalinate it. Desalinated water is also expensive. So poor regions cannot benefit from this technology. For coastal cities, however, desalination is increasingly viewed as a competitive choice.
In 2023, Israel was using desalination to replenish the Sea of Galilee's water supply.
Environmental concerns
Intake
Water intake structures cause adverse environmental impact by sucking fish and shellfish or their eggs into an industrial system. There, the organisms may be killed or injured by heat, physical stress, or chemicals. Larger organisms may be killed or injured when they become trapped against screens at the front of an intake structure. Alternative intake types that mitigate these impacts include beach wells, but they require more energy and higher costs.
The Kwinana Desalination Plant opened in the Australian city of Perth, in 2007. Water there and at Queensland's Gold Coast Desalination Plant and Sydney's Kurnell Desalination Plant is withdrawn at 0.1 m/s (0.33 ft/s), which is slow enough to let fish escape. The plant provides nearly 140,000 m3 (4,900,000 cu ft) of clean water per day.
Outflow
Desalination processes produce large quantities of brine, possibly at above ambient temperature, and contain residues of pretreatment and cleaning chemicals, their reaction byproducts and heavy metals due to corrosion (especially in thermal-based plants).
Brine is denser than seawater and therefore sinks to the ocean bottom and can damage the ecosystem. Brine dispersal from the desalination plants has been seen to travel several kilometers away, meaning that it has the potential to cause harm to ecosystems far away from the plants. Careful reintroduction with appropriate measures and environmental studies can minimize this problem.
Health aspects
Desalination removes iodine from water and could increase the risk of iodine deficiency disorders.
Methods
There are several methods. Each has advantages and disadvantages.
Vacuum distillation
The traditional process used in these operations is vacuum distillation—essentially boiling it to leave impurities behind. In desalination, atmospheric pressure is reduced, thus lowering the required temperature needed. Liquids boil when the vapor pressure equals the ambient pressure and vapor pressure increases with temperature. Thus, because of the reduced temperature, low-temperature "waste" heat from electrical power generation or industrial processes can be employed.
Multi-stage flash distillation
Water is evaporated and separated from sea water through multi-stage flash distillation, which is a series of flash evaporations. Each subsequent flash process utilizes energy released from the condensation of the water vapor from the previous step and so on.
Multiple-effect distillation
Multiple-effect distillation (MED) works through a series of steps called “effects”. Incoming water is sprayed onto vertically or, more commonly, horizontally oriented pipes which are then heated to generate steam. The steam is then used to heat the next batch of incoming sea water. To increase efficiency, the steam used to heat the sea water can be taken from nearby power plants. Although this method is the most thermodynamically efficient, a few limitations exist such as a max temperature and max number of effects.
Vapor-compression distillation
Vapor-compression evaporation involves using either a mechanical compressor or a jet stream to compress the vapor present above the liquid. The compressed vapor is then used to provide the heat needed for the evaporation of the rest of the sea water. Since this system only requires power, it is more efficient if kept at a small scale.
Reverse osmosis
The principal competing process uses membranes to desalt saline water, principally applying reverse osmosis (RO). The RO membrane processes use semipermeable membranes and applied pressure (on the membrane feed side) to preferentially induce water permeation through the membrane while rejecting salts. Reverse osmosis plant membrane systems typically use less energy than thermal desalination processes. Desalination processes are driven by either thermal (e.g., distillation) or electrical (e.g., RO) as the primary energy types. Energy cost in desalination processes varies considerably depending on water salinity, plant size and process type. At present the cost of seawater desalination, for example, is higher than traditional water sources, but it is expected that costs will continue to decrease with technology improvements that include, but are not limited to, reduction in plants footprint, improvements to plant operation and optimization, more effective feed pretreatment, and lower cost energy sources.
The Reverse Osmosis process is not maintenance free. Various factors interfere with efficiency: ionic contamination (calcium, magnesium etc.); DOC; bacteria; viruses; colloids & insoluble particulates; biofouling and scaling. In extreme cases destroying the RO membranes. To mitigate damage, various pretreatment stages are introduced. Anti-scaling inhibitors include acids and other agents like the organic polymers Polyacrylamide and Polymaleic Acid), Phosphonates and Polyphosphates. Inhibitors for fouling are biocides (as oxidants against bacteria and viruses), like chlorine, ozone, sodium or calcium hypochlorite. At regular intervals, depending on the membrane contamination; fluctuating seawater conditions; or prompted by monitoring processes the membranes need to be cleaned, known as emergency or shock-flushing. Flushing is done with inhibitors in a fresh water solution. Thus the system needs to go offline. This procedure is environmental risky, since contaminated water is rejected into the ocean without treatment. Sensitive marine habitats can be irreversibly damaged.
Freeze-thaw
Freeze-thaw desalination uses freezing to remove fresh water from frozen seawater.
One method, invented by Alexander Zarchin, used freezing and vacuuming of salt from seawater.
Solar evaporation
Solar evaporation mimics the natural water cycle, in which the sun heats the sea water enough for evaporation to occur. After evaporation, the water vapor is condensed onto a cool surface.
Electrodialysis reversal
Electrodialysis utilizes electric potential to move the salts through a membrane.
Costs
Factors that determine the costs for desalination include capacity and type of facility, location, feed water, labor, energy, financing and concentrate disposal. Costs of desalinating sea water (infrastructure, energy, and maintenance) are generally higher than fresh water from rivers or groundwater, water recycling, and water conservation, but alternatives are not always available. Desalination costs in 2013 ranged from US$0.45 to US$1.00/m3. More than half of the cost comes directly from energy cost, and since energy prices are very volatile, actual costs can vary substantially.
The cost of untreated fresh water in the developing world can reach US$5/cubic metre.
| Method | Cost (US$/liter) |
|---|---|
| Passive solar ( 30.42% energy efficient) | 0.034 |
| Passive solar (improved single-slope, India) | 0.024 |
| Passive solar (improved double slope, India) | 0.007 |
| Multi Stage Flash (MSF) | < 0.001 |
| Reverse Osmosis (Concentrated solar power) | 0.0008 |
| Reverse Osmosis (Photovoltaic power) | 0.000825 |
| Area | Consumption Litre/person/day |
Desalinated Water Cost US$/person/day |
|---|---|---|
| US | 378 | 0.38 |
| Europe | 189 | 0.19 |
| Africa | 57 | 0.06 |
| UN recommended minimum | 49 | 0.05 |
Desalination stills control pressure, temperature and brine concentrations to optimize efficiency. Nuclear-powered desalination might be economical on a large scale.
In 2014, the Israeli facilities of Hadera, Palmahim, Ashkelon, and Sorek were desalinizing water for less than US$0.40 per cubic meter. As of 2006, Singapore was desalinating water for US$0.49 per cubic meter.
Images for kids
-
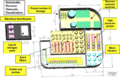
Schematic representation of a typical desalination plant using reverse osmosis
-
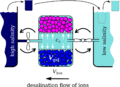
Scheme of the desalination machine: the desalination box of volume V_{box} contains a gel of volume V_{gel} which is separated by a sieve from the outer solution volume V_{out} = V_{box} - V_{gel}. The box is connected to two big tanks with high and low salinity by two taps which can be opened and closed as desired. The chain of buckets expresses the fresh water consumption followed by refilling the low-salinity reservoir by salt water.
See also
 In Spanish: Desalinización para niños
In Spanish: Desalinización para niños