Aluminium antimonide facts for kids
Quick facts for kids Aluminium antimonide |
|
|---|---|
 |
|
| Identifiers | |
| CAS number | |
| PubChem | |
| SMILES | [Al]#[Sb] |
|
InChI
InChI=1/Al.Sb/rAlSb/c1-2
|
|
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | black crystals |
| Density | 4.26 g/cm3 |
| Melting point | |
| Boiling point | |
| insoluble | |
| Band gap | 1.58 eV |
| Refractive index (nD) | 3.3 |
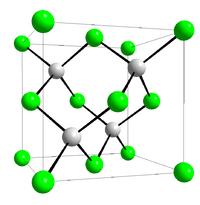
| Structure | |
| Crystal structure | Zinc blende |
| Space group | T2d-F-43m |
| Coordination geometry |
Tetrahedral |
| Hazards | |
| MSDS | MSDS |
| NFPA 704 |
|
| Autoignition temperature |
590 °C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Aluminium antimonide is a chemical compound. Its chemical formula is AlSb. It has aluminium and antimonide ions in it. Compounds like it, such as gallium antimonide and indium antimonide can be made into LEDs and detectors for Infrared radiation but aluminium antimonide doesn't work as well. It is used instead to make the other compounds work better by stopping defects forming as they are made.
Properties
Aluminium antimonide is a black solid. It is a semiconductor. It reacts with acids to make stibine. It is a reducing agent. It has properties between a salt and an alloy.
Related pages
See also
 In Spanish: Antimoniuro de aluminio para niños
In Spanish: Antimoniuro de aluminio para niños

All content from Kiddle encyclopedia articles (including the article images and facts) can be freely used under Attribution-ShareAlike license, unless stated otherwise. Cite this article:
Aluminium antimonide Facts for Kids. Kiddle Encyclopedia.

