Aluminium chloride facts for kids
Quick facts for kids Aluminium chloride |
|
|---|---|
 |
|
 |
|
| IUPAC name | aluminium chloride |
| Other names | aluminium(III) chloride aluminum trichloride |
| Identifiers | |
| CAS number | |
| PubChem | |
| ChEBI | CHEBI:30114 |
| RTECS number | BD0530000 |
| SMILES | Cl[Al](Cl)Cl |
|
InChI
InChI=1/Al.3ClH/h;3*1H/q+3;;;/p-3
|
|
| Gmelin Reference | 1876 |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | white or pale yellow solid, hygroscopic |
| Density | 2.48 g/cm3 (anhydrous) 2.398 g/cm3 (hexahydrate) |
| Melting point | |
| Boiling point | |
| 439 g/l (0 °C) 449 g/l (10 °C) 458 g/l (20 °C) 466 g/l (30 °C) 473 g/l (40 °C) 481 g/l (60 °C) 486 g/l (80 °C) 490 g/l (100 °C) |
|
| Solubility | soluble in hydrogen chloride, ethanol, chloroform, carbon tetrachloride slightly soluble in benzene |
| Vapor pressure | 133.3 Pa (99 °C) 13.3 kPa (151 °C) |
| Viscosity | 0.35 cP (197 °C) 0.26 cP (237 °C) |
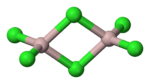
| Structure | |
| Crystal structure | Monoclinic, mS16 |
| Space group | C12/m1, No. 12 |
| Lattice constant | a = 0.591 nm, b = 0.591 nm, c = 1.752 nm |
| Coordination geometry |
Octahedral (solid) Tetrahedral (liquid) |
| Molecular shape | Trigonal planar (monomeric vapour) |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−704.2 kJ/mol |
| Standard molar entropy S |
109.3 J/mol·K |
| Specific heat capacity, C | 91.1 J/mol·K |
| Pharmacology | |
| ATC code | |
| Hazards | |
| NFPA 704 |
|
| U.S. Permissible exposure limit (PEL) |
none |
| Related compounds | |
| Other anions | Aluminium fluoride Aluminium bromide Aluminium iodide |
| Other cations | Boron trichloride Gallium trichloride Indium(III) chloride Magnesium chloride |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Aluminium chloride (AlCl3), is a chemical compound. It is a white or yellow crystalline solid. It melts at a low temperature. It is made by reacting aluminium oxide with hydrochloric acid. The anhydrous (without water) form may be made by reacting aluminium and chlorine. It is used in the making of chemicals. It is also used in deodorants. It can cause slight irritation.
Related pages
See also
 In Spanish: Cloruro de aluminio para niños
In Spanish: Cloruro de aluminio para niños

All content from Kiddle encyclopedia articles (including the article images and facts) can be freely used under Attribution-ShareAlike license, unless stated otherwise. Cite this article:
Aluminium chloride Facts for Kids. Kiddle Encyclopedia.

