Absolute temperature facts for kids
Absolute temperature, also called thermodynamic temperature, is the temperature of an object on a scale where 0 is taken as absolute zero. Absolute temperature scales are Kelvin (degree units Celsius) and Rankine (degree unit Fahrenheit).
Absolute zero is the temperature at which a system is in the state of lowest possible (minimum) energy. As molecules approach this temperature their movements drop towards zero. It is the lowest temperature a gas thermometer can measure. No electronic devices work at this temperature. The Kinetic energy of the molecules becomes negligible or zero.
Examples
Common temperatures in the absolute scale are:
- 0 °C (freezing point of water) = 273.15 K
- 25 °C (room temperature) = 298.15 K
- 100 °C (boiling point of water) = 373.15 K
- 0K (absolute zero) = - 273.15 Celsius
- 233.15K (equal measures in Celsius and Fahrenheit)=-40 Celsius
- Triple point of water= 273.16K (equal measure in Celsius) 0.01°c.
Conversion
To convert from the Celsius scale into the absolute temperature, you add 273.15 and change °C to K. To get a temperature on the absolute scale to the Celsius scale, subtract 273.15 and change K to °C. This is normally used in the science world. Kelvin is used globally as a part of the International System of Units. It is one of the 7 base units of the system. The value of Absolute temperature is 0K.
- Celsius to Kelvin: K=C+273.15
- Kelvin to Celsius: C=K-273.15
- Fahrenheit to Rankine: R=F+459.67
- Rankine to Fahrenheit: F=R-459.67
Images for kids
-
Figure 1 The translational motion of fundamental particles of nature such as atoms and molecules are directly related to temperature. Here, the size of helium atoms relative to their spacing is shown to scale under 1950 atmospheres of pressure. These room-temperature atoms have a certain average speed (slowed down here two trillion-fold). At any given instant however, a particular helium atom may be moving much faster than average while another may be nearly motionless. Five atoms are colored red to facilitate following their motions. This animation illustrates statistical mechanics, which is the science of how the group behavior of a large collection of microscopic objects emerges from the kinetic properties of each individual object.
-
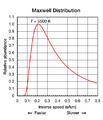
Figure 2 The translational motions of helium atoms occur across a range of speeds. Compare the shape of this curve to that of a Planck curve in Fig. 5 below.
-
Figure 4 The temperature-induced translational motion of particles in solids takes the form of phonons. Shown here are phonons with identical amplitudes but with wavelengths ranging from 2 to 12 average inter-molecule separations (a).
-
Figure 8 When many of the chemical elements, such as the noble gases and platinum-group metals, freeze to a solid — the most ordered state of matter — their crystal structures have a close-packed arrangement. This yields the greatest possible packing density and the lowest energy state.
-
Figure 9 Due to the effects of zero-point energy, helium at ambient pressure remains a superfluid even when exceedingly close to absolute zero; it won't freeze unless under 25 bar of pressure (~25 atmospheres).