Uranium trioxide facts for kids
Quick facts for kids Uranium trioxide |
|
|---|---|
 |
|
| IUPAC name | Uranium trioxide Uranium(VI) oxide |
| Other names | Uranyl oxide Uranic oxide |
| Identifiers | |
| CAS number | |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | yellow-orange powder |
| Density | 5.5–8.7 g/cm3 |
| Melting point | |
| Partially soluble | |
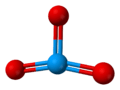
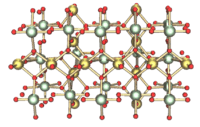
| Structure | |
| Crystal structure | see text |
| Space group | I41/amd (γ-UO3) |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−1230 kJ·mol−1 |
| Standard molar entropy S |
99 J·mol−1·K−1 |
| Hazards | |
| EU classification | Very toxic (T+) Dangerous for the environment (N) |
| NFPA 704 |
|
| R-phrases | R26/28, R33, R51/53 |
| S-phrases | (S1/2), S20/21, S45, S61 |
| Flash point | Non-flammable |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Uranium trioxide (UO3), also called uranyl oxide, uranium(VI) oxide, and uranic oxide, is an oxide of uranium. UO3 is a hexavalent oxide, which means that the uranium in the compound has an oxidation state of +6. It can be formed by heating uranyl nitrate to 400 °C.
UO3 is a poisonous and slightly radioactive substance. It can be harmful if it is breathed in, ingested, or makes contact with skin.
Images for kids

All content from Kiddle encyclopedia articles (including the article images and facts) can be freely used under Attribution-ShareAlike license, unless stated otherwise. Cite this article:
Uranium trioxide Facts for Kids. Kiddle Encyclopedia.