Third law of thermodynamics facts for kids
The third law of thermodynamics says:
- If an object reaches the absolute zero of temperature (0 K = −273.15C = −459.67 °F), its atoms will stop moving.
The definition is: at absolute zero , the entropy of a perfectly crystalline substance is zero.
Experimentally, it is not possible to obtain −273.15°C, as of now. It is found that most of the gases either liquify or solidify before reaching such a temperature, gaseous molecules no longer remaining. So far, scientists have been able to get close to, but not exactly, absolute zero. This may change in the future.
Related pages
Images for kids
-
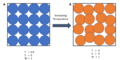
a) Single possible configuration for a system at absolute zero, i.e., only one microstate is accessible. Thus S = k ln W = 0. b) At temperatures greater than absolute zero, multiple microstates are accessible due to atomic vibration (exaggerated in the figure). Since the number of accessible microstates is greater than 1, S = k ln W > 0.
See also
 In Spanish: Tercer principio de la termodinámica para niños
In Spanish: Tercer principio de la termodinámica para niños