Raoult's law facts for kids
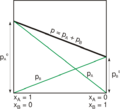
Simply put, Raoult's law states that the vapour pressure, of a binary solution, containing a non-volatile solute, is directly related to the mole fraction of solvent (i.e. volatile) in the solution.
Also, it states that the vapour pressure of each component in a binary solution containing volatile components is directly related to its respective mole fraction in the solution.
Relative going-down of vapour pressure is equal to mole fraction of non volatile and non-electrolytic solute. This is known as Raoult's law.
P-P(INITIAL)/P(INITIAL)=n(SOLUTE)/n(SOLVENT)
Images for kids
See also
 In Spanish: Ley de Raoult para niños
In Spanish: Ley de Raoult para niños

All content from Kiddle encyclopedia articles (including the article images and facts) can be freely used under Attribution-ShareAlike license, unless stated otherwise. Cite this article:
Raoult's law Facts for Kids. Kiddle Encyclopedia.