Perchlorate facts for kids
Quick facts for kids Perchlorate |
|
|---|---|
 |
|
|
Perchlorate
|
|
| Identifiers | |
| CAS number | |
| PubChem | |
| DrugBank | DB03138 |
| MeSH | |
| ChEBI | CHEBI:49706 |
| SMILES | [O-][Cl+3]([O-])([O-])[O-] |
| Gmelin Reference | 2136 |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Conjugate acid | Perchloric acid |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
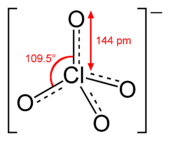
A perchlorate is a chemical compound containing the perchlorate ion, ClO−
4. The majority of perchlorates are commercially produced salts. They are mainly used for propellants, exploiting properties as powerful oxidizing agents and to control static electricity in food packaging. Perchlorate contamination in food, water, and other parts of the environment has been studied in the U.S. because of its harmful effects on human health. Perchlorate reduces hormone production in the thyroid gland.
Most perchlorates are colorless solids that are soluble in water. Four perchlorates are of primary commercial interest: ammonium perchlorate (NH4ClO4), perchloric acid (HClO4), potassium perchlorate (KClO4), and sodium perchlorate (NaClO4). Perchlorate is the anion resulting from the dissociation of perchloric acid and its salts upon their dissolution in water. Many perchlorate salts are soluble in non-aqueous solutions.
Related pages
See also
 In Spanish: Perclorato para niños
In Spanish: Perclorato para niños