Osteocyte facts for kids
Quick facts for kids Osteocyte |
|
|---|---|
 |
|
| Transverse section of a bone | |
 |
|
| Illustration showing a single osteocyte | |
| Latin | osteocytus |
An osteocyte, an oblate shaped type of bone cell with dendritic processes, is the most commonly found cell in mature bone. It can live as long as the organism itself. The adult human body has about 42 billion of them. Osteocytes do not divide and have an average half life of 25 years. They are derived from osteoprogenitor cells, some of which differentiate into active osteoblasts (which may further differentiate to osteocytes). Osteoblasts/osteocytes develop in mesenchyme.
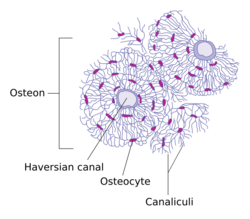
In mature bones, osteocytes and their processes reside inside spaces called lacunae (Latin for a pit) and canaliculi, respectively. Osteocytes are simply osteoblasts trapped in the matrix that they secrete. They are networked to each other via long cytoplasmic extensions that occupy tiny canals called canaliculi, which are used for exchange of nutrients and waste through gap junctions.
Although osteocytes have reduced synthetic activity and (like osteoblasts) are not capable of mitotic division, they are actively involved in the routine turnover of bony matrix, through various mechanosensory mechanisms. They destroy bone through a rapid, transient (relative to osteoclasts) mechanism called osteocytic osteolysis. Hydroxyapatite, calcium carbonate and calcium phosphate is deposited around the cell.
Contents
Structure
Osteocytes have a stellate shape, approximately 7 micrometers deep and wide by 15 micrometers in length. The cell body varies in size from 5–20 micrometers in diameter and contain 40–60 cell processes per cell, with a cell to cell distance between 20–30 micrometers. A mature osteocyte contains a single nucleus that is located toward the vascular side and has one or two nucleoli and a membrane. The cell also exhibits a reduced size endoplasmic reticulum, Golgi apparatus and mitochondria, and cell processes that radiate largely towards the bone surfaces in circumferential lamellae, or towards a haversian canal and outer cement line typical of osteons in concentric lamellar bone. Osteocytes form an extensive lacunocanalicular network within the mineralized collagen type I matrix, with cell bodies residing within lacunae, and cell/dendritic processes within channels called canaliculi.
Development
The fossil record shows that osteocytes were present in bones of jawless fish 400 to 250 million years ago. Osteocyte size has been shown to covary with genome size; and this relationship has been used in paleogenomic research.
During bone formation, an osteoblast is left behind and buried in the bone matrix as an "osteoid osteocyte", which maintains contact with other osteoblasts through extended cellular processes. The process of osteocytogenesis is largely unknown, but the following molecules have been shown to play a crucial role in the production of healthy osteocytes, either in correct numbers or specific distributions: matrix metalloproteinases (MMPs), dentin matrix protein 1 (DMP-1), osteoblast/osteocyte factor 45 (OF45), Klotho, TGF-beta inducible factor (TIEG), lysophosphatidic acid (LPA), E11 antigen, and oxygen. 10–20% of osteoblasts differentiate into osteocytes. Those osteoblasts on the bone surface that are destined for burial as osteocytes slow down matrix production, and are buried by neighboring osteoblasts that continue to produce matrix actively.
Palumbo et al. (1990) distinguish three cell types from osteoblast to mature osteocyte: type I preosteocyte (osteoblastic osteocyte), type II preosteocyte (osteoid osteocyte), and type III preosteocyte (partially surrounded by mineral matrix). The embedded "osteoid-osteocyte" must do two functions simultaneously: regulate mineralization and form connective dendritic processes, which requires cleavage of collagen and other matrix molecules. The transformation from motile osteoblast to entrapped osteocyte takes about three days, and during this time, the cell produces a volume of extracellular matrix three times its own cellular volume, which results in 70% volume reduction in the mature osteocyte cell body compared to the original osteoblast volume. The cell undergoes a dramatic transformation from a polygonal shape to a cell that extends dendrites toward the mineralizing front, followed by dendrites that extend to either the vascular space or bone surface. As the osteoblast transitions to an osteocyte, alkaline phosphatase is reduced, and casein kinase II is elevated, as is osteocalcin.
Osteocytes appear to be enriched in proteins that are resistant to hypoxia, which appears to be due to their embedded location and restricted oxygen supply. Oxygen tension may regulate the differentiation of osteoblasts into osteocytes, and osteocyte hypoxia may play a role in disuse-mediated bone resorption.
Function
Although osteocytes are relatively inert cells, they are capable of molecular synthesis and modification, as well as transmission of signals over long distances, in a way similar to the nervous system. They are the most common cell type in bone (31,900 per cubic millimeter in bovine bone to 93,200 per cubic millimeter in rat bone). Most of the receptor activities that play an important role in bone function are present in the mature osteocyte.
Osteocytes are an important regulator of bone mass. Osteocytes contain glutamate transporters that produce nerve growth factors after bone fracture, evidence of a sensing and information transfer system. When osteocytes were experimentally destroyed, the bones showed a significant increase in bone resorption, decreased bone formation, trabecular bone loss, and loss of response to unloading.
Osteocytes are mechanosensor cells that control the activity of osteoblasts and osteoclasts within a basic multicellular unit (BMU), a temporary anatomic structure where bone remodeling occurs. Osteocytes generate an inhibitory signal that is passed through their cell processes to osteoblasts for recruitment to enable bone formation.
Osteocytes are also a key endocrine regulator in the metabolism of minerals such as phosphates. Osteocyte-specific proteins such as sclerostin have been shown to function in mineral metabolism.
See also
- List of human cell types derived from the germ layers
- List of distinct cell types in the adult human body


