Mixtures and solutions facts for kids
Mixtures and solutions are what is created when you combine various substances together.
Contents
What's the difference between a mixture and a solution?
A mixture is not the same throughout. Its a combination of two or more substances that don't chemically combine together. For example, a mixture of sand and water. You can see the difference between the sand and the water just by looking at it. They both keep their individual properties.
A solution is the same throughout. So you can't see any different properties. If you mix sugar and water together, the sugar dissolves and it creates a new product called a solution. They are also homogeneous, which means the substances are evenly distributed through the solution. No matter where you take a sample, there will be the same amount of water and sugar in the sample as any other sample taken anywhere else in the solution.
Mixtures

In chemistry, a mixture is a substance that is made up of two or more simpler substances. These substances can be chemical elements or compounds. A mixture can be made of liquids, solids, or gases.
A mixture is not the same as a compound which is made of two or more atoms connected together. For instance, a mixture of the gases hydrogen and nitrogen contains hydrogen and nitrogen, not the compound ammonia which is made of hydrogen and nitrogen atoms.
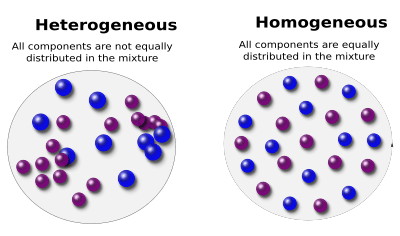
A mixture where the different parts can be distinguished easily is called heterogenous, one where this is not the case is called homogeneous.
A third form is called colloid. A colloid may be a mixture of one substance that may spread out evenly inside another substance. They may be in two different phases or states of matter.
If one substance in a mixture dissolves in the other, it is called a solution. For example, if sugar is put in water it forms a mixture, then dissolves to make a solution. If it does not dissolve, it would be called a suspension.
Solids can be mixtures also. Alloys are mixtures. Many kinds of soil and rock are mixtures of different minerals. Thus, a mixture is made of two or more elements and/or compounds which are not chemically combined.
Heterogeneous and homogeneous mixtures
Heterogeneous mixture examples
Homogeneous mixture examples
-
Red wine in a glass, a homogeneous mixture.
Solutions
Making a saline water solution by dissolving table salt (NaCl) in water. The salt is the solute and the water the solvent.
In chemistry, a solution is a homogeneous mixture of two or more substances. The substances that are dissolved are called solutes. The substance the solutes are dissolved in is called the solvent. An example from everyday experience is a solid like salt or sugar (which are crystalline solids), dissolved in a liquid (like water). Gases can dissolve in liquids. An example is carbon dioxide or oxygen in water. Liquids may dissolve in other liquids and gases in other gases.
The amount of solute added to the solvent determines the concentration of the solution. The solution with the large amount of solute is called a concentrated solution; the solution with less solute is called a dilute solution.
Examples of solid solutions are alloys and some minerals. For example, brass is an alloy of copper and zinc.
Related pages