Electrowinning facts for kids
Electrowinning is a manufacturing process for making metals from a solution containing dissolved metal. The process involves passing an electrical current from an inert electrode through the solution containing the metal, to plate the metal to another electrode. Also called electroplating, it is a common process for producing metals like copper, cobalt, nickel, zinc, aluminum, and gold among others. Electrowinning is one of the last processes in metal production. It commonly follows leaching which is a process that dissolves minerals into a solution.
Contents
Process
The electrical current is passed from the inert electrode, called the anode, and passes through the metal containing solution, called the electrolyte, and the metal is plated on another electrode, called the cathode. The ability to plate a metal depends on the reduction potential. The reduction potential depends on the many factors such as the metal in question, the solution temperature, the pH of the solution and other factors. The rate at which the metal can be produced depends on the amount of current being passed through the solution.
Industry
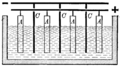
Industrially the process is made up of a square cathode with equal sized anodes on each side so that the metal can be plated evenly on both sides of the cathode. A cell consists of alternating anodes and cathodes, starting and ending with anodes. A production plant consists of many cells allowing for production of lots of metal.
Images for kids
-
Electrorefining technology converting spent commercial nuclear fuel into metal.
See also
 In Spanish: Electrodeposición para niños
In Spanish: Electrodeposición para niños