Electron crystallography facts for kids
Electron crystallography is a method to determine the arrangement of atoms in solids using a transmission electron microscope (TEM).
This method works in many cases where X-ray crystallography does not. The latter needs large 3-D crystals to work.
Protein structures are usually done from 2-dimensional crystals (sheets or helices), polyhedrons such as viral capsids, or dispersed proteins. Electrons can be used in these situations, whereas X-rays cannot, because electrons interact more strongly with atoms than X-rays do.
Electron crystallography is a method to determine the arrangement of atoms in solids using a transmission electron microscope (TEM). The method was invented by Aaron Klug, who won the Nobel Prize in Chemistry for this, and his studies on virus structures and transfer RNA, in 1982.
The first electron crystallographic protein structure to be solved was bacteriorhodopsin in 1990. Since then, several other high-resolution structures have been done by electron crystallography, including the light-harvesting complex, and the bacterial flagellum.
Related pages
Images for kids
-
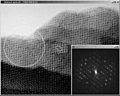
Electron microscopy image of an inorganic tantalum oxide, with its Fourier transform, inset. Notice how the appearance changes from the upper thin region to the thicker lower region. The unit cell of this compound is about 15 by 25 Ångström. It is outlined at the centre of the figure, inside the result from image processing, where the symmetry has been taken into account. The black dots show clearly all the tantalum atoms. The diffraction extends to 6 orders along the 15 Å direction and 10 orders in the perpendicular direction. Thus the resolution of the EM image is 2.5 Å (15/6 or 25/10). This calculated Fourier transform contain both amplitudes (as seen) and phases (not displayed).