Döbereiner's lamp facts for kids
Döbereiner's lamp is one of the first lighters. It was developed in 1823 by the German chemist Johann Wolfgang Döbereiner. The lighter was produced until around 1880. The Heidelberg Castle and also the Deutsches Museum (German Museum) still have original Döbereiner lamps to show.
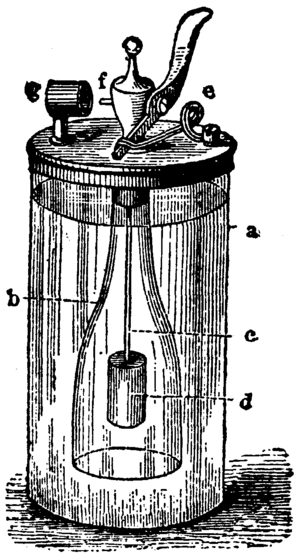
The lighter works based on a chemical reaction between hydrogen and oxygen. In the glass cylinder (a in the picture on the right) is sulfuric acid. In the open bottle (b) is zinc and at the top is a valve. With the lever (f), the bottle will be opened. Then, the sulfuric acid flows into the bottle. When it reaches the Zinc, a reaction starts. It produces hydrogen.
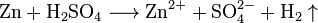
The hydrogen can go out of the bottle at the top. Next to the valve, there is platinum. The hydrogen gas has to bypass the platinum. The hydrogen and the oxygen from the air react because of the catalytic effect of platinum. The gas mixture (called oxyhydrogen) then burns, because the reaction is exothermic, and produces water.

To stop this reaction, the bottle only needs to be closed, that means the lever needs to be released. The hydrogen can no longer go out of the bottle and pushes the sulfuric acid back into the glass cylinder.