Calcium nitrate facts for kids
Quick facts for kids Calcium nitrate |
|
|---|---|
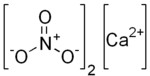 |
|
 |
|
| Other names | Kalksalpeter, nitrocalcite, Norwegian saltpeter, lime nitrate |
| Identifiers | |
| CAS number | |
| PubChem | |
| RTECS number | EW2985000 |
| SMILES | [Ca+2].[O-][N+]([O-])=O.[O-][N+]([O-])=O |
|
InChI
InChI=1/Ca.2NO3/c;2*2-1(3)4/q+2;2*-1
|
|
| Properties | |
| Molecular formula | Ca(NO3)2 |
| Molar mass | 164.088 g/mol (anhydrous) 236.15 g/mol (tetrahydrate) |
| Appearance | colorless solid hygroscopic |
| Density | 2.504 g/cm3 (anhydrous) 1.896 g/cm3 (tetrahydrate) |
| Melting point |
561 °C, 834 K, 1042 °F |
| Boiling point |
decomposes (anhydrous) |
| Solubility in water | anhydrous: 1212 g/L (20 °C) 2710 g/L (40 °C) tetrahydrate: 1050 g/L (0 °C) 1290 g/L (20 °C) 3630 g/L (100 °C) |
| Solubility | soluble in ammonia almost insoluble in nitric acid |
| Solubility in ethanol | 51.4 g/100 g (20 °C) 62.9 g/100 g (40 °C) |
| Solubility in methanol | 134 g/100 g (10 °C) 144 g/100 g (40 °C) 158 g/100 g (60 °C) |
| Solubility in acetone | 16.8 g/kg (20 °C) |
| Acidity (pKa) | 6.0 |
| -45.9·10−6 cm3/mol | |
| Structure | |
| Crystal structure | cubic (anhydrous) monoclinic (tetrahydrate) |
| Hazards | |
| NFPA 704 |
|
| R-phrases | R22, R41 |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions | Calcium sulfate Calcium chloride |
| Other cations | Magnesium nitrate Strontium nitrate Barium nitrate |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Calcium nitrate, also known as Norwegian saltpeter, is a chemical compound. Its chemical formula is Ca(NO3)2. It contains calcium and nitrate ions.
Properties
Calcium nitrate is a colorless solid. It is an oxidizing agent. It absorbs water from the air. It decomposes to make nitrogen dioxide and calcium oxide when heated.
Preparation
Calcium nitrate is made by reacting calcium hydroxide or calcium carbonate with nitric acid. It is made when nitric acid reacts with calcium phosphate. It can be made by reacting ammonium nitrate and calcium hydroxide.
Uses
Calcium nitrate is used in fertilizers to add nitrogen to the soil. It can be complexed with urea to make a different nitrogen fertilizer.
Related pages
Images for kids
See also
 In Spanish: Nitrato de calcio para niños
In Spanish: Nitrato de calcio para niños



