Boyle's law facts for kids
Boyle's law, also referred to as the Boyle–Mariotte law, or Mariotte's law (especially in France), is an experimental gas law that describes how the pressure of a gas tends to increase as the volume of the container decreases.
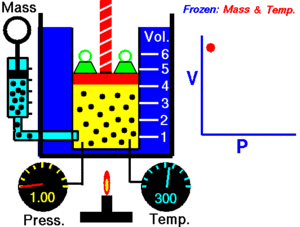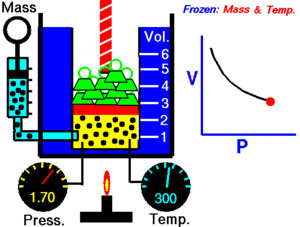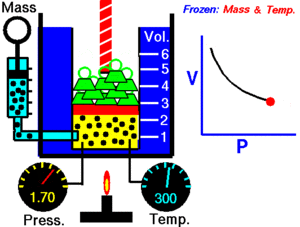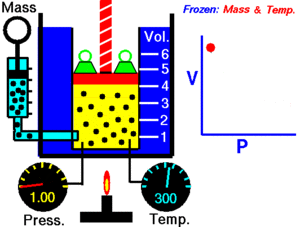
For a given mass of gas at a constant temperature, the product of the pressure and the volume is constant. As the volume decreases, the pressure increases in proportion, and vice versa. For example, when the pressure halves, the volume doubles.
Suppose you have a tank that contains a certain volume of gas at a certain pressure. When you decrease the volume of the tank, the same number of gas particles is now contained in a smaller space. Therefore, the number of collisions increases. Therefore, the pressure is greater.
The law was found by Robert Boyle in 1662, and afterwards independently by Edme Mariotte in 1679.
See also
 In Spanish: Ley de Boyle-Mariotte para niños
In Spanish: Ley de Boyle-Mariotte para niños