Alkane facts for kids
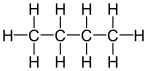
Alkanes are chemical compounds, made of carbon and hydrogen. The simplest alkane is methane, which is made of one carbon atom and four hydrogen atoms. Larger alkanes have two or more carbon atoms connected or bonded together in a chain. The carbon atoms of alkanes are joined together by single bonds, which is what makes them different from alkenes. The alkane's general formula is CnH2n+2.
These are some alkanes:
|
Production
Alkanes come from crude oil. Crude oil is a natural resource, a thick black fluid found by drilling underground. Crude oil is a mixture of alkanes of different chain length - composed of different numbers of carbon atoms.
Fractional distillation is the method used to separate the different hydrocarbons in crude oil, used to get a purer or more distilled, sample of a single alkane.
Images for kids
-
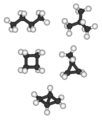
C4 alkanes and cycloalkanes (left to right): n-butane and isobutane are the two C4H10 isomers; cyclobutane and methylcyclopropane are the two C4H8 alkane isomers. Bicyclo[1.1.0]butane is the only C4H6 alkane and has no alkane isomer; tetrahedrane (below) is the only C4H4 alkane and so has no alkane isomer.
-
Monobromination of propane
-
Extraction of oil, which contains many distinct hydrocarbons including alkanes
-
Methanogenic archaea in the gut of this cow are responsible for some of the methane in Earth's atmosphere.
-
An oil refinery at Martinez, California.
See also
 In Spanish: Alcano para niños
In Spanish: Alcano para niños