Alessandro Volta facts for kids
Quick facts for kids
Alessandro Volta
|
|
|---|---|
 |
|
| Born | 18 February 1745 |
| Died | 5 March 1827 (aged 82) Como, Lombardy-Venetia, Austrian Empire
|
| Nationality | Italian |
| Known for |
|
| Awards |
|
| Scientific career | |
| Fields | Physics and chemistry |
Alessandro Giuseppe Antonio Anastasio Volta ( 18 February 1745 – 5 March 1827) was an Italian physicist and chemist who was a pioneer of electricity and power who is credited as the inventor of the electric battery and the discoverer of methane. He invented the voltaic pile in 1799, and reported the results of his experiments in 1800 in a two-part letter to the president of the Royal Society. With this invention Volta proved that electricity could be generated chemically and debunked the prevalent theory that electricity was generated solely by living beings. Volta's invention sparked a great amount of scientific excitement and led others to conduct similar experiments, which eventually led to the development of the field of electrochemistry.
Volta also drew admiration from Napoleon Bonaparte for his invention, and was invited to the Institute of France to demonstrate his invention to the members of the institute. Volta enjoyed a certain amount of closeness with the emperor throughout his life and he was conferred numerous honours by him. Volta held the chair of experimental physics at the University of Pavia for nearly 40 years and was widely idolised by his students.
Despite his professional success, Volta tended to be a person inclined towards domestic life and this was more apparent in his later years. At this time he tended to live secluded from public life and more for the sake of his family until his eventual death in 1827 from a series of illnesses which began in 1823. The SI unit of electric potential is named in his honour as the volt.
Contents
Early life and works
Volta was born in Como, a town in northern Italy, on 18 February 1745. In 1794, Volta married an aristocratic lady also from Como, Teresa Peregrini, with whom he raised three sons: Zanino, Flaminio, and Luigi. His father, Filippo Volta, was of noble lineage. His mother, Donna Maddalena, came from the family of the Inzaghis.
In 1774, he became a professor of physics at the Royal School in Como. A year later, he improved and popularised the electrophorus, a device that produced static electricity. His promotion of it was so extensive that he is often credited with its invention, even though a machine operating on the same principle was described in 1762 by the Swedish experimenter Johan Wilcke. In 1777, he travelled through Switzerland, where he befriended H. B. de Saussure.
In the years between 1776 and 1778, Volta studied the chemistry of gases. He researched and discovered methane after reading a paper by Benjamin Franklin of the United States on "flammable air". In November 1776, he found methane in the marshes of Angera on Lake Maggiore, and by 1778 he managed to isolate methane. He devised experiments such as the ignition of methane by an electric spark in a closed vessel.
Volta also studied what we now call electrical capacitance, developing separate means to study both electrical potential difference (V) and charge (Q), and discovering that for a given object, they are proportional. This is called Volta's Law of Capacitance, and for this work the unit of electrical potential has been named the volt.
In 1779, he became a professor of experimental physics at the University of Pavia, a chair that he occupied for almost 40 years. Volta's lectures were so crowded with students that the subsequent emperor Joseph II ordered the construction (based on a project by Leopold Pollack) of a new "physical theater", today the "Aula Volta". Furthermore, the emperor granted Volta substantial funding to equip the physics cabinet with instruments, purchased by Volta in England and France. At the University History Museum of the University of Pavia there are 150 of them, used by Alessandro Volta.
Volta and Galvani
Luigi Galvani, an Italian physicist, discovered something he named, "animal electricity" when two different metals were connected in series with a frog's leg and to one another. Volta realised that the frog's leg served as both a conductor of electricity (what we would now call an electrolyte) and as a detector of electricity. He also understood that the frog's legs were irrelevant to the electric current, which was caused by the two differing metals. He replaced the frog's leg with brine-soaked paper, and detected the flow of electricity by other means familiar to him from his previous studies. In this way he discovered the electrochemical series, and the law that the electromotive force (emf) of a galvanic cell, consisting of a pair of metal electrodes separated by electrolyte, is the difference between their two electrode potentials (thus, two identical electrodes and a common electrolyte give zero net emf). This may be called Volta's Law of the electrochemical series.
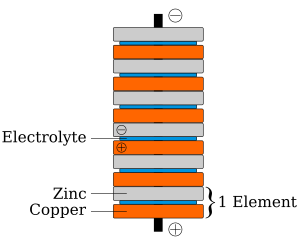
In 1800, as the result of a professional disagreement over the galvanic response advocated by Galvani, Volta invented the voltaic pile, an early electric battery, which produced a steady electric current. Volta had determined that the most effective pair of dissimilar metals to produce electricity was zinc and copper. Initially he experimented with individual cells in series, each cell being a wine goblet filled with brine into which the two dissimilar electrodes were dipped. The voltaic pile replaced the goblets with cardboard soaked in brine.
Early battery
In announcing his discovery of the voltaic pile, Volta paid tribute to the influences of William Nicholson, Tiberius Cavallo, and Abraham Bennet.
The battery made by Volta is credited as one of the first electrochemical cells. It consists of two electrodes: one made of zinc, the other of copper. The electrolyte is either sulfuric acid mixed with water or a form of saltwater brine. The electrolyte exists in the form 2 H+
and SO2−
4. Zinc metal, which is higher in the electrochemical series than both copper and hydrogen, is oxidized to zinc cations (Zn2+) and creates electrons that move to the copper electrode. The positively charged hydrogen ions (protons) capture electrons from the copper electrode, forming bubbles of hydrogen gas, H2. This makes the zinc rod the negative electrode and the copper rod the positive electrode. Thus, there are two terminals, and an electric current will flow if they are connected. The chemical reactions in this voltaic cell are as follows:
- Zinc:
- Zn → Zn2+ + 2e−
- Zn → Zn2+ + 2e−
- Sulfuric acid:
- 2H+
+ 2e−
→ H
2
- 2H+
Copper metal does not react, but rather it functions as a catalyst for the hydrogen-gas formation and an electrode for the electric current. The sulfate anion (SO2−
4) does not undergo any chemical reaction either, but migrates to the zinc anode to compensate for the charge of the zinc cations formed there. However, this cell also has some disadvantages. It is unsafe to handle, since sulfuric acid, even if diluted, can be hazardous. Also, the power of the cell diminishes over time because the hydrogen gas is not released. Instead, it accumulates on the surface of the copper electrode and forms a barrier between the metal and the electrolyte solution.
Last years and retirement
In 1809, Volta became associated member of the Royal Institute of the Netherlands. In honour of his work, Volta was made a count by Napoleon Bonaparte in 1810.
Volta retired in 1819 to his estate in Camnago, a frazione of Como, Italy, now named "Camnago Volta" in his honour. He died there on 5 March 1827, just after his 82nd birthday. Volta's remains were buried in Camnago Volta.
Legacy
Volta's legacy is celebrated by the Tempio Voltiano memorial located in the public gardens by the lake. There is also a museum that has been built in his honour, which exhibits some of the equipment that Volta used to conduct experiments. Nearby stands the Villa Olmo, which houses the Voltian Foundation, an organization promoting scientific activities. Volta carried out his experimental studies and produced his first inventions near Como. In the Old Campus of the University of Pavia there is the classroom (Aula Volta) commissioned by Emperor Joseph II to Leopoldo Pollack in 1787 for the lectures of Alessandro Volta, while in the University History Museum there are many scientific instruments that belonged to Volta and his chair and his blackboard.
His image was depicted on the Italian Lire 10,000 note (1990–1997) along with a sketch of his voltaic pile.
In late 2017, Nvidia announced a new workstation-focused GPU microarchitecture called Volta.
The electric eel species Electrophorus voltai, described in 2019 and the strongest bioelectricity producer in nature, was named after Volta.
Images for kids
-
Volta explains the principle of the "electric column" to Napoleon in 1801
See also
 In Spanish: Alessandro Volta para niños
In Spanish: Alessandro Volta para niños
- Armstrong effect
- Eudiometer
- History of the battery
- History of the internal combustion engine
- Lemon battery
- Mercury beating heart
- Thermoelectric effect
- Volta pistol
- Volta potential
- Volta (lunar crater)
- Volta Prize